
The purpose of our review of southeastern aquatic reptiles is to provide a benchmark on population status and trends as we currently understand them. In the future, ecologists and conservation biologists may be able to evaluate whether improvements have been made in the status of these species or if society has allowed continued declines.
For this review, we define the southeastern United States as encompassing all of North Carolina, South Carolina, Georgia, Florida, Alabama, Mississippi, Louisiana, Arkansas, Tennessee, and Kentucky. In addition, we include taxa from the Coastal Plain of Virginia, the Tennessee River drainages of western Virginia, and the "boot heel" (Mississippi Lowlands) region of southeastern Missouri.
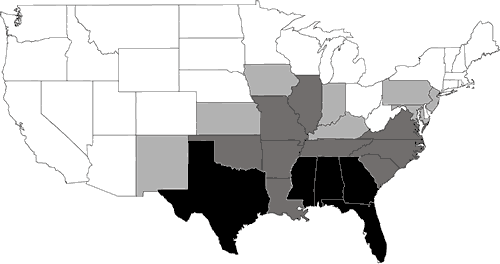
Nearly 200 species of reptiles inhabit the United States and Canada in the form of turtles, snakes, lizards, and crocodilians. Clearly, the Southeast is a region where reptiles are in great abundance (Figure 1). Because of its wealth of wetland habitats, the Southeast is also the historical home of most of the aquatic reptiles in North America (Figure 2). The southeastern U.S. contains the greatest diversity of freshwater turtles (Harless and Morlock, 1979; Iverson, 1992) with up to 17 species occurring in one area (Bury, 1979; Figure 3). Twenty-three species of aquatic turtles are known from Alabama (Lydeard and Mayden, 1995). In comparison, fewer than ten species are found throughout the Northeast, with no more than eight occurring together (Figure 3). Only one aquatic turtle species is native to the Pacific coastal region. The Southeast also contains 17 aquatic snake species (Ernst and Barbour, 1989). In contrast, only four species of aquatic snakes are found in the Northeast, three of which also occur in the Southeast.
Generally, aquatic reptiles can be grouped into one of three categories (Table
1). Permanently aquatic species are those that spend almost their entire time
in the water (i.e., they only leave water to nest or bask). Most river turtles
such as Graptemys ssp., Macroclemys temminckii, and Sternotherus
minor are in this category. Semi-aquatic species inhabit wetland habitats
but may engage in overland travel, hibernate on land, or forage terrestrially.
Some species of turtles (e.g., Clemmys guttata and Deirochelys reticularia)
and snakes (Agkistrodon piscivorous and most Nerodia ssp.) fit
this category. Marginally aquatic species live primarily on land but are often
common in the vicinity of wetland habitats. These include the snakes Thamnophis
sauritus and Crotalus horridus atricaudatus and the lizards Ophisaurus
ventralis and Eumeces anthracinus. We acknowledge that these categories
are somewhat arbitrary and other herpetologists might choose to classify several
of the species differently.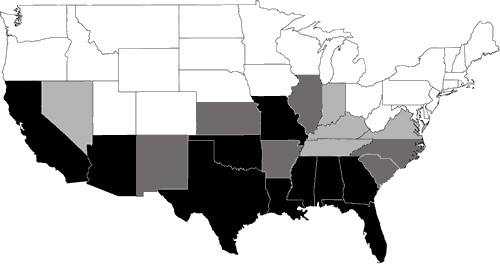
For the purposes of determining "imperiled" status as an operational category for this review, we have relied heavily on the ranking system designed by The Nature Conservancy and used by all state Natural Heritage Programs (Table 2). Natural Heritage Program methodology requires that each taxon be given a global rank (G1-G5), where G1 is extremely rare throughout the range of the taxon and G5 denotes very common. Likewise, each state assigns a state rank (S1-S5) to all taxa within its boundary. For example, in Virginia, the chicken turtle (D. reticularia) is ranked G5S1 indicating the species’ perceived common status due to its wide global range (G5) but very limited occurrence in Virginia (S1). The yellow-blotched sawback turtle (Graptemys flavimaculata) has a rank of G2 (global) and S2 (state) indicating its restricted global occurrence in one river system in Mississippi.
From approximately 1725 until the middle 1800s, numerous prominent naturalists described the fauna and flora of North America (Adler, 1979). Although we have been able to gain an appreciation of the natural landscape from early botanists, information on the abundances and habitats of reptiles is sparse. Early emphasis on the herpetofauna of North America focused on descriptive taxonomy and any available population data are largely anecdotal (McIlhenny, 1935; Ditmars, 1936; Dellinger and Black, 1938; Cagle, 1953; Martof, 1956). For example, an early American naturalist and conservationist from Louisiana, E. A. McIlhenny (1872-1949), wrote in 1935 (McIlhenny, 1935; page 116), "alligators are not in immediate danger [of extinction], but it is extremely doubtful if they ever again will be an attractive feature of our waterways, as they were during the latter part of the last century." Ditmars (1936; page 4) stated, "Very large alligators are so rare nowadays that a specimen twelve feet long must be considered a giant. There was a time in Florida — long since gone — when alligators fourteen and fifteen feet long were no great rarity." Rarely, the early naturalists wrote about perceived declines of other animals. In Florida, as early as the 1930s, snake populations were regarded as declining due to proliferation of "motor roads" and the spread of fires (Ditmars, 1939).
Table 1. Numbers of imperiled aquatic reptile species exhibiting various associations with aquatic environments within the southeastern United States. |
|||||
Aquatic Association 1 |
Taxa |
||||
Turtles |
Snakes |
Lizards |
Crocodilians |
Totals |
|
Permanently aquatic |
19 |
8 |
0 |
0 |
27 |
Semi-aquatic |
7 |
5 |
0 |
2 |
14 |
Marginally aquatic |
0 |
4 |
2 |
0 |
6 |
Totals |
26 |
17 |
2 |
2 |
47 |
1 For definitions of aquatic associations see text. |
|||||
When historical information was available on individual species, we added it to the Species Accounts section of this chapter. However, making definitive statements about population trends was difficult because of the frequent anecdotal nature of most of the historical data. Furthermore, modern survey techniques have shown some animals to be more abundant than previously thought by early naturalists, and have led to a better understanding of habitat and behavior.
Since European settlement 400 years ago, the landscape of the southeastern United States has been a progressive alteration of terrestrial and aquatic ecosystems. A large percentage of the Coastal Plain physiographic region was originally dominated by fire-maintained longleaf pine (Pinus palustris) communities. Between 1773 and 1777, William Bartram explored the Carolinas, Georgia, and Florida (Bartram [1791] 1980). He described virgin forests of oaks, hickories, yellow poplar, and pine, as well as wetlands and open savannas. On several occasions he traveled by horse through forests of huge spreading live oaks with open understories. Bartram described viewing an oval wetland (probably a Carolina bay) with a large expanse of grassy marsh on one side and a forest of towering cypress on the other in which numerous parrots (presumably the extinct Carolina parakeets) foraged. Coastal Plain rivers were bordered by forests of bottomland hardwoods and bald cypress (Taxodium distichum). These meandering rivers with their sandy bottoms were filled with tree snags (Benke, 1990).
Unfortunately, the majority of the longleaf pine forest has been logged, replanted in monocultures of loblolly or slash pine, and managed on 20-year rotations. Lands supporting longleaf pine-wiregrass (Aristida stricta) or longleaf pine-bluestem (Andropogon sp.) had declined to less than one-sixth of their original extent by 1946 and to less than ten percent today (Frost et al., 1986). Other previously forested lands are now in agriculture or have been urbanized. The loss of forest cover is illustrated in stark reality when one examines Landsat photographs. For example, the forested 300 square mile (777 km2) Savannah River Site (SRS), in South Carolina, is easily recognizable on a photograph that encompasses an expanse including New Jersey and Florida. The SRS is clearly distinguishable from the surrounding predominantly agricultural landscape.
Table 2. Definitions of Natural Heritage State Rarity Ranks. Analogous Global Ranks (G1-G5, GU) would have the same definitions except that they would refer to rarity throughout the entire range of a species. Ranks should not be interpreted as legal designations. |
|
Rank |
Definition |
S1 |
extremely rare, usually five or fewer occurrences in state; very vulnerable to extirpation |
S2 |
very rare; usually between 6 and 20 occurrences in state; susceptible to extirpation |
S3 |
rare to uncommon; usually between 21 and 100 occurrences in state; may be vulnerable to large scale disturbances |
S4 |
uncommon to common; usually more than 100 occurrences in state; not usually vulnerable to immediate threats |
S5 |
very common; demonstrably secure under present conditions |
SU |
status uncertain; this status is often warranted because of insufficient data associated with low search effort or cryptic nature of the species |
Many of the natural Coastal Plain wetlands have been filled, ditched and drained, or left isolated in a fragmented landscape. In Virginia alone during 1950-1970, 57 percent of the state’s freshwater wetlands were lost: 45 percent to agriculture, 27 percent to development, and the remainder to man-made ponds, reservoirs, and other causes (Tiner, 1987). Hefner and Brown (1984) reported wetland losses in the Southeast at a rate of about 385,471 acres per year (156,000 ha per year), with a total loss of about 7.4 million acres (3 million ha), 84 percent of the national total (Richardson and Gibbons, 1993).
The majority of bottomland hardwood forests were logged during the last century
and 400-year-old cypress swamps have been replaced with red maple, sweetgum,
and blackgum woods. Cypress does not regenerate well on sites that have been
both logged and burned (Gunderson, 1984). Bottomland floodplain forests in the
Southeast have also been destroyed incrementally through conversion to agriculture
and diking for flood control (Sharitz and Mitsch, 1993). Sharitz and Gibbons
(1982) provided detailed descriptions of the logging and draining of such huge
tracts as Great Dismal Swamp in Virginia and Green Swamp in North Carolina.
Gosselink and Lee (1989) reported an overall loss of 69 percent of bottomland
hardwood wetlands throughout the continental U.S. since European settlement.
The original extent of southern bottomland hardwood forests was thought to be
17.7 million acres (about 7.2 million ha); the current extent is 6.6 million
acres (about 2.7 million ha), a 63 percent decline. MacDonald et al. (1979)
estimated a 78 percent loss of forested wetlands in the Mississippi River floodplain.
Fifty percent of Louisiana’s forested wetlands have been lost (Turner and Craig,
1980). 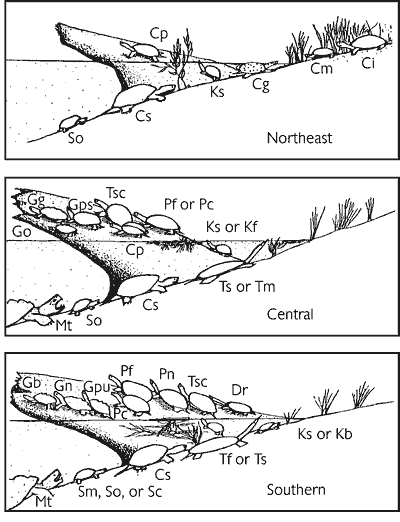
The facilitation of navigation has resulted in channelization and de-snagging operations of most large Coastal Plain rivers. Benke (1990) noted that although humans have been modifying streams and rivers for centuries, major alterations really began in the 1930s with water projects for flood control, navigation, water supply, and electricity. In his review, Benke (1990) reported that only 42 free-flowing rivers greater than 124 miles (200 km) in length remained in the continental U.S. The Southeast has the greatest remaining length of high quality rivers but also the least amount of government protection (Benke, 1990).
On the Piedmont of Georgia, the oak-hickory-loblolly pine forests have been harvested several times (Skeen et al., 1993). Although the last century saw most of this region used for cotton and tobacco, recent uses have included short-term timber rotation and urbanization. As a result of inappropriate farming and logging practices, most of the original topsoil has been lost, leaving the red clay subsoil to erode into streams and rivers. Piedmont rivers were once gravel- and cobble-bottomed and flowed clear. They currently have mud bottoms and flow red-brown with silt and sediments.
Mountain forests have been logged and streams and rivers impacted by runoff, siltation, and pollution. The once majestic American chestnut-dominated forests are gone, the result of a fungus that was imported with ornamental trees around 1900. Chestnut accounted for 50 to 80 percent of the canopy in forests in the Smoky Mountains (Shelford, 1963). Runoff from mining operations has polluted many biologically diverse streams, especially in the Cumberland Plateau physiographic region, and is associated with declines in fish and freshwater mollusk faunas (Williams et al., 1993). Mountain bogs and fens in the Appalachian region have been ditched, drained, and otherwise impacted by farming and cattle grazing.
In summary, the landscape today is vastly different from the one Europeans encountered 400 years ago. The disappearance and modification of natural habitats is correlated with declines of the natural biodiversity, including aquatic reptiles.
This section does not treat all taxa of aquatic reptiles in the southeastern United States. To be included, a taxon had to have a Natural Heritage Program (NHP) rank of S1, S2, or S3 in at least one of the southeastern states in which it occurs or be protected at the state or federal level (e.g., endangered, threatened, special concern, candidate). A designation of candidate (C) by the U.S. Fish and Wildlife Service indicates that enough information exists to support proposals to list the particular species as threatened or endangered and future listing is anticipated. Candidate species (C) were formerly Category 1 (C1) species, while those species formerly listed as Category 2 (C2) no longer have an official federal designation (U.S. Federal Register, 1996). Any taxon that was ranked as S4 or S5 was considered not to be of conservation concern or not to be imperiled at this time. By using this approach, our species counts can be used as benchmarks to gauge future declines or improvements in the status of imperiled species. For each species discussed, The Nature Conservancy’s global (G) rank is given following the first use of the scientific name.
American alligator — Alligator mississippiensis — G5
The American alligator is federally listed as threatened due to similarity of appearance with the American crocodile. Although once threatened with global extinction, alligator populations have increased concomitantly with protection measures, such as a ban on alligator products and regulated hunting. In Arkansas, the alligator is ranked S3 and the distribution of the animal has been altered due to stocking programs (Arkansas Natural Heritage Program, unpublished data). In North Carolina, the alligator is ranked S3 and is state listed as threatened.
American crocodile — Crocodylus acutus — G2
The American crocodile is federally listed as endangered. The Florida Natural Areas Inventory ranks the American crocodile as S1 and it is state listed as endangered. The American crocodile was confirmed as occurring in Florida in 1875. Between then and 1936, 70 specimens were collected between Lake Worth and Cape Sable (Ditmars, 1936). To protect this species, anti-poaching laws need to be enforced, habitat encroachment needs to be reduced, and mortality associated with road traffic needs to be lowered. King et al. (1982) estimated the Florida population at 200 to 500 individuals. Currently, fewer than 200 to 400 animals, including 25 breeding females, remain in Everglades National Park and Crocodile Lake National Wildlife Refuge.
Eastern glass lizard — Ophisaurus ventralis — G5
The eastern glass lizard is ranked S1 and listed as threatened in Virginia, where it is found in coastal grassy freshwater swales between dunes and is a peripheral species at the northern edge of its range (Mitchell and Pague, 1991). We have found eastern glass lizards around the borders of Carolina bay wetlands in South Carolina. Martof (1956) considered them uncommon in Georgia. Louisiana ranks this lizard as S2 (Louisiana Natural Heritage Program, 1996).
Coal skink — Eumeces anthracinus — G5
The southern coal skink (Eumeces anthracinus pluvialis) is ranked S3 in Florida and is included in this review because it is marginally aquatic. It occurs around the borders of acidic wetlands in pine flatwoods and has been observed to retreat into shallow water and hide under bottom rubble (Means, 1992).
Northern water snake — Nerodia sipedon — G5
The Carolina salt marsh snake (Nerodia sipedon williamengelsi) is ranked S3 and is listed as special concern in North Carolina. It is limited to salt marshes of the southern Outer Banks and Pamlico and Core sounds in North Carolina (H. LeGrand, North Carolina Natural Heritage Program, pers. comm.).
Southern water snake — Nerodia fasciata — G5
The broad-banded water snake (Nerodia fasciata confluens) is ranked S1 and state listed as endangered in Kentucky, at the northern periphery of its range. Wetland drainage and destruction are causing the decline of this subspecies (Kentucky Nature Preserves Commission, unpublished data).
Salt marsh snake — Nerodia clarkii — G4
The U.S. Fish and Wildlife Service (USFWS) formerly listed the salt marsh snake (Nerodia clarkii) as C2. The Gulf salt marsh snake (Nerodia clarkii clarkii), ranked S3 in Florida, inhabits brackish coastal habitats on the Gulf Coast and is threatened by degradation of habitat, including development, and oil spills. Insecticides, such as those used in mosquito control, may be affecting the Gulf salt marsh snake (Florida Natural Areas Inventory, unpublished data). The Gulf salt marsh snake is ranked S2S3 in Mississippi and surveys are currently being conducted (T. Mann, Mississippi Natural Heritage Program, pers. comm.). The Gulf salt marsh snake is ranked S1S2 in Alabama (Alabama Natural Heritage Program, 1996). The Atlantic salt marsh snake (N. c. taeniata) is ranked S1 in Florida and is listed as threatened by the USFWS and the state of Florida (Kochman and Christman, 1992; Florida Natural Areas Inventory, 1995). Atlantic salt marsh snakes only occur in Volusia County, on the Atlantic coast in Florida, and require pristine brackish and salt marshes (Florida Natural Areas Inventory, unpublished data).
Plainbellied water snake — Nerodia erythrogaster — G5
Recently, the USFWS proposed the copperbellied water snake (Nerodia erythrogaster neglecta) as threatened (U.S. Fish and Wildlife Service, 1993). The copperbellied water snake is ranked SU and is state listed as threatened in Tennessee, where only five localities for its presence are known (Tennessee Division of Natural Heritage, unpublished data). Populations in Tennessee display traits that may indicate intergradation with the yellowbellied water snake (N. e. flavigaster) (Tennessee Division of Natural Heritage, 1996). The copperbellied water snake is a northern subspecies that occurs in disjunct colonies throughout its range. Major threats to the subspecies include the drainage of wetlands and agricultural activities, primarily in Tennessee, Kentucky, Illinois, Indiana, and Ohio. Kentucky ranks the copperbellied water snake as S2S3 and lists it as special concern. Only 14 occurrences are known in Kentucky, and wetland destruction is considered the cause of this snake’s decline (Kentucky Nature Preserves Commission, unpublished data).
Mississippi green water snake — Nerodia cyclopion — G5
Sparse population information is available for the Mississippi green water snake. Ditmars (1936) considered the snake rare in the Mississippi Valley. The Nature Conservancy ranks it as G5, yet most states within its range consider it to be rare (Florida S1; Arkansas S2; Alabama S2; Tennessee S1; Kentucky S1). Missouri listed the Mississippi green water snake as endangered as a consequence of drastic reductions in cypress swamps (Johnson, 1987). No recent confirmations for the species have been reported in Missouri where the snake is presently listed as extirpated (Missouri Natural Heritage Database, 1995). The Mississippi green water snake is near the northern edge of its range in Kentucky, with the drainage of wetlands and agricultural runoff contributing to its decline (Kentucky Nature Preserves Commission, unpublished data). The Mississippi green water snake is ranked S1 and considered in need of management in Tennessee (Tennessee Division of Natural Heritage, 1996). The Mississippi green water snake was considered abundant in Louisiana by Dundee and Rossman (1989).
Florida green water snake — Nerodia floridana — G5
The Florida green water snake is ranked S2 and considered a species of concern in South Carolina. The species exhibits a much greater level of habitat specificity than other water snakes, seeming to prefer ponds with water lilies and relatively permanent water (S. Bennett, South Carolina Heritage Trust, pers. comm.). The two known South Carolina populations occur on the Savannah River Site and the Charleston Naval Weapons Station. These snakes seem slow to recolonize wetlands that have dried, making them susceptible to local extinctions (Seigel et al., 1995). Alabama ranks the Florida green water snake as SU (Alabama Natural Heritage Program, 1996). The species is ranked S2 in Georgia where it seems to prefer prairies in the Okefenokee Swamp, sinkhole lakes, and Carolina bays with open canopies (Georgia Natural Heritage Program, unpublished data). Martof (1956) considered this snake uncommon to common in Georgia. The South Carolina populations appear to be disjunct from the Georgia populations by several hundred kilometers (Conant and Collins, 1991), but the reason for this gap is unclear. Using allozyme electrophoresis, Thompson (1994) found no differences between the South Carolina and nearest Georgia populations.
Striped crayfish snake — Regina alleni — G5
The striped crayfish snake is ranked S2 in Georgia, with the northernmost distributional records being from the Okefenokee Swamp. Godley (1980) determined that the striped crayfish snake is active at night, feeding on crayfish, dragonfly nymphs, and dwarf sirens (Pseudobranchus striatus). Ditmars (1939) considered the animal rare, a designation possibly linked to this snake’s nocturnal behavior which makes it poorly known.
Crayfish snake — Regina rigida — G5
Crayfish snakes occur around pond edges in vegetation, in freshwater tidal marshes, in floodplains, and in flatwoods ponds where they eat crayfish. The Gulf crayfish snake (R. r. sinicola) is ranked S2 in Arkansas and S3 in Mississippi. The glossy crayfish snake (R. r. rigida) is ranked S1 in Virginia and is known only from one disjunct population in a seemingly pristine freshwater tidal marsh (Richmond, 1940; Buhlmann et al., 1993b). In North Carolina, where it is known from marshes and cypress ponds (North Carolina Natural Heritage Program, unpublished data), the glossy crayfish snake is ranked S3. Ditmars (1939) considered this snake rare because it was infrequently encountered. Martof (1956) considered the glossy crayfish snake uncommon in Georgia.
Graham’s crayfish snake — Regina grahamii — G5
Although poorly known, Graham’s crayfish snake has been found along the margins of ponds, streams, sloughs, bayous, and swamps (Neill, 1958; Conant and Collins, 1991). This snake is ranked S2 in Arkansas where populations are believed to be declining because of the removal of tree snags, channelization of streams, and destruction of wetland habitat (Arkansas Natural Heritage Program, unpublished data).
Queen snake — Regina septemvittata — G5
Populations of the queen snake (Regina septemvittata) in the Boston Mountains of Arkansas are disjunct from the remainder of the species’ range (Conant and Collins, 1991). The USFWS previously listed this disjunct population as C2, and it is ranked S1 in Arkansas (Arkansas Natural Heritage Commission, 1996). Ernst and Barbour (1989) stated that queen snakes prefer clean, unpolluted streams with abundant crayfish populations. They also suggested that snake populations may be reduced as a result of water pollution and acid rain, which affects crayfish populations. In Arkansas, most streams inhabited by queen snakes exhibit water pollution from cattle pastures, poultry operations, and human occupation (Trauth, 1991).
Kirtland’s snake — Clonophis kirtlandi — G2
The USFWS formerly listed Kirtland’s snake as C2. Kirtland’s snake may be declining throughout its range due to the drainage of prairie marshland over the last two centuries (Kentucky Nature Preserves Commission, unpublished data). This northern and midwestern species reaches the southern periphery of its range in Kentucky, where it is ranked S1 and is state listed as endangered (Kentucky Nature Preserves Commission, 1995). The Kentucky Nature Preserves Commission has five historic records from 1946-1968 and one verified record in the 1980s. Ditmars (1936; page 172) stated, "in some districts the species is as abundant as the garter snake."
Black swamp snake — Seminatrix pygaea — G5
The black swamp snake is an inhabitant of thick vegetation in ponds and sluggish streams. The Carolina swamp snake (Seminatrix pygaea paludis) is ranked SU and considered a species of concern in South Carolina. It is ranked S3 in North Carolina. The North Florida swamp snake (S. p. pygaea) is ranked S3 in Georgia and populations are likely becoming more disjunct as habitat between cypress ponds and other wetlands becomes increasingly fragmented. The North Florida swamp snake is ranked S2 in Alabama.
Eastern ribbon snake — Thamnophis sauritus — G5
The Florida Natural Areas Inventory Program recognizes a unique population of the peninsula ribbon snake (Thamnophis sauritus sackeni) in the lower Florida Keys in which individuals possess a yellow to orange mid-dorsal stripe. This population is ranked S1 and is state listed as threatened (Florida Natural Areas Inventory, 1995). Protection of the remaining permanent freshwater ponds on the lower Florida Keys will be necessary for this population to persist (Weaver and Christman, 1992). The eastern ribbon snake (T. s. sauritus) is ranked S2S3 and listed as special concern in Kentucky where it is declining due to loss of wetland habitat (Kentucky Nature Preserves Commission, unpublished data).
Western ribbon snake — Thamnophis proximus — G5
The western ribbon snake (Thamnophis proximus proximus) reaches the most eastern periphery of its range in Kentucky where it is ranked S1S2 and state listed as threatened. Population declines in Kentucky are attributed to wetland losses (Kentucky Nature Preserves Commission, unpublished data).
Rainbow snake — Farancia erythrogramma — G5
The South Florida rainbow snake (Farancia erythrogramma seminola) is ranked S1 in Florida and represents a disjunct taxon known only from three specimens collected in 1949 and 1952 in one creek in Glades County, Florida (Moler, 1992). The South Florida rainbow snake, if it still exists, is probably threatened as a result of pollution from agricultural runoff. The eastern rainbow snake (F. e. erythrogramma) is ranked S2 and is state listed as endangered in Mississippi. Declines are believed due to channelization, siltation, and pollution (Mississippi Department of Fisheries, Wildlife, and Parks, 1992). Other states consider the eastern rainbow snake to be marginally rare (Georgia S3, Alabama S3, Louisiana S2). Habitat degradation of small streams and the draining of wetlands as well as a peripheral distribution (Louisiana) are cited as causes of concern or reasons for the species’ rarity (S. Shively, Louisiana Natural Heritage Program, pers. comm.). Martof (1956) considered them uncommon in Georgia. Mitchell (1994) considered the species secure in Virginia despite its spotty occurrence.
Mud snake — Farancia abacura — G5
The western mud snake (Farancia abacura reinwardtii) is ranked S3 and listed as special concern in Kentucky where it reaches the northeastern periphery of its range. Declines are probably the result of wetland drainage, logging, and channelization in the western Kentucky coal field area (Kentucky Nature Preserves Commission, unpublished data). Missouri lists the western mud snake as a "watchlist" species because of its peripheral occurrence at the northern edge of its range (Missouri Natural Heritage Database, 1995). Ditmars (1939) found these snakes most abundant in cypress swamps of the Gulf states.
Canebrake rattlesnake — Crotalus horridus atricaudatus — G5
The canebrake rattlesnake is ranked S1 and state listed as endangered in Virginia (Roble, 1996). Declines have been attributed to direct killing, destruction of forested wetlands, and an ever-expanding road network (Mitchell, 1994). Persecution by humans has resulted in declines of this snake throughout its range. Based on recent research using radio-telemetry, large tracts of land are required by this species (A. L. Savitzky, Old Dominion University, pers. comm.). Florida ranks the canebrake rattlesnake as S3 (Florida Natural Areas Inventory, 1995).
Alligator snapping turtle — Macroclemys temminckii — G3G4
The USFWS formerly listed the alligator snapping turtle as C2, and the turtle is now a species of concern in every state within its range. Ditmars (1936) found alligator snapping turtles common in the Mississippi River, as did Martof (1956) in Georgia. However, Pritchard (1989) documented the extensiveness of the alligator snapping turtle fishery and the effect that unregulated harvest has had on this slow-to-mature, long-lived organism. Arkansas ranks the alligator snapping turtle as SU, and anecdotal information suggests that most harvested individuals are being taken to the Louisiana meat market (Pritchard, 1989). Florida ranks the alligator snapping turtle as S3 and attributes declines to habitat degradation and direct harvest (Florida Natural Areas Inventory, unpublished data). Mississippi ranks the turtle as S3, and state law prohibits a commercial fishery for it. However, an illegal fishery exists with the catch going to Louisiana, and alligator snapping turtles in Mississippi also incur mortality in the poorly regulated trotline fishery (T. Mann, Mississippi Natural Heritage Program, pers. comm.). Georgia populations have suffered from commercial exploitation, as have Louisiana and Alabama populations (Pritchard, 1989). Tennessee populations might be declining due to dredging and the channelization of streams (Tennessee Division of Natural Heritage, unpublished data). Other state rankings are as follows: Georgia S3, state listed as threatened; Alabama S3, state listed as protected; Kentucky S1S2, state listed as threatened; Louisiana S3; Missouri S1, state listed as rare.
Stripe-necked musk turtle — Sternotherus minor — G5
The stripe-necked musk turtle (Sternotherus minor peltifer) is ranked S2 in Virginia. North Carolina ranks it as S1 and lists it as a species of special concern. In those states, it is limited to streams of the upper Tennessee River drainage. These streams, which once harbored some of the most diverse mollusk faunas in the world, have been impacted by fly ash spills, heavy metals, acid mine drainage, and sewage. Stripe-necked musk turtles have heavy crushing jaws and feed on freshwater mussels and snails. They will likely continue to decline as their food source disappears. Stripe-necked musk turtles reach the western periphery of their range in Louisiana, where they are ranked S1.
Razorback musk turtle — Sternotherus carinatus — G5
The razorback musk turtle is ranked S2 in Arkansas and its continued existence requires the maintenance of aquatic systems of the Coastal Plain, including riverine pools and slow-moving streams (Arkansas Natural Heritage Program, unpublished data). The razorback musk turtle is ranked S1 in Alabama, due to its recent discovery in the Alabama portion of the Escatawpa River (Blankenship et. al., 1995).
Flattened musk turtle — Sternotherus depressus — G2
The flattened musk turtle is federally listed as threatened (U.S. Fish and Wildlife Service, 1992). Alabama ranks this turtle as S2 and lists it as protected. The species seems to be doing well in only a few streams and is declining or non-existent in most of its historic range (M. Bailey, Alabama Natural Heritage Program, pers. comm.). The flattened musk turtle is an Alabama endemic that is confined to permanent streams of the upper Black Warrior River system above the Fall Line (Mount, 1975; Mount, 1981; U.S. Fish and Wildlife Service, 1992). The food of flattened musk turtles consists primarily of mollusks (Mount, 1981). Historically, the Black Warrior River system supported 45 species of unionid mussels. A 1975 survey found 18 species, and a 1990 survey by the USFWS found only two species (U.S. Fish and Wildlife Service, 1992). Six major impoundments have been built along the river and water quality degradation as a result of row-crop agricultural activity, timbering, urban drainage, poultry farms, feedlots, and coal mines has been documented (Dodd, 1990). Locust Fork, the largest and longest Black Warrior tributary, once contained the bulk of the S. depressus populations. Locust Fork is now degraded along its entire length as a result of heavy siltation associated with mines, although some turtles still occur there (K. Dodd, National Biological Service, pers. comm.). The flattened musk turtle is also threatened by disease and collection for the pet trade (Dodd, in press).
Striped mud turtle — Kinosternon baurii — G5
The striped mud turtle is ranked SU and considered of special concern in South Carolina. In Virginia (S4), North Carolina (S3), and Georgia (S3), the species appears restricted to high quality riverine floodplain forests and blackwater cypress swamps and streams, and it is not found in man-made habitats or silted wetlands. In Florida, a unique population called the Key mud turtle is ranked S2 and occurs in permanent and temporary freshwater ponds on some islands of the lower Florida Keys. Rapid development is depleting the water table and thus threatening these ponds. Florida lists this population as endangered (Dunson, 1992).
Common map turtle — Graptemys geographica — G5
Although widespread in its distribution, the common map turtle is in a peripheral portion of its range in southwest Virginia where it is ranked S2S3 (Mitchell, 1994; Roble, 1996). The species is found in the same streams and subjected to the same threats as Sternotherus minor peltifer. The common map turtle is also in a peripheral portion of its range in northwest Georgia, where it is known only from the Conasauga River. Georgia ranks the common map turtle as S1 and lists it as rare (Georgia Natural Heritage Program, 1996). These turtles are associated with moving water systems. Human activities have dammed many rivers, removed the tree snags used for basking, and dredged the bottoms that harbor the mollusks that comprise a large portion of the diet of map turtles. Although the time has passed for us to know with certainty what historical population levels were, most ecologists familiar with map turtles would generally concur that their numbers have decreased and that most populations now appear on a downward trend.
Barbour’s map turtle — Graptemys barbouri — G2
The USFWS formerly listed Barbour’s map turtle as C2. Barbour’s map turtle is endemic to the Appalachicola River system. This range includes the Appalachicola in Florida, the Flint River in Georgia, and the Chattahoochee River in Georgia and Alabama. In Florida, where this species is ranked S2 and listed as special concern, the largest populations are found in alluvial and spring-fed rivers, such as the Chipola, and other clear, limestone-bottomed streams with an abundance of mollusks and fallen trees (Florida Natural Areas Inventory, unpublished data). In Georgia, Barbour’s map turtle is also ranked S2 and is state listed as threatened. It occurs in the Flint River upstream to Lake Blackshear, where individuals are being affected by an unknown shell disease. Lovich et al. (1996) suggested that exposure to toxic chemicals may be causing the observed shell dermatitis. Other basking species in Lake Blackshear, notably Trachemys scripta and Pseudemys concinna are also similarly afflicted, suggesting a relationship between the causal agent and shell drying. In the Chattahoochee River along the Georgia-Alabama line, threats include channelization, dredging, and pollution that affects both the turtles and mollusks. Collection for the pet trade has also been heavy. Alabama ranks Barbour’s map turtle as S2 and lists it as protected. Martof (1956) considered Barbour’s map turtle uncommon to common in Georgia.
Alabama map turtle — Graptemys pulchra — G4
The Alabama map turtle is ranked S2 in Mississippi and is declining in numbers due to degraded riverine water quality in the Tombigbee River system (T. Mann, Mississippi Natural Heritage Program, pers. comm.). It is in a peripheral portion of its range in northwest Georgia, where it is only known from the Conasauga River (Santhuff and Wilson, 1990). Georgia ranks it as S1 and lists it as rare. The global range of the Alabama map turtle is confined to the Tombigbee and Alabama river systems that empty into Mobile Bay.
Pascagoula map turtle — Graptemys gibbonsi — G3
The Pascagoula map turtle was recently described as a new species and was previously considered to be a variant of Graptemys pulchra (Lovich and McCoy, 1992). The species is confined to large to medium-sized rivers of the Pascagoula and Pearl river systems, including the Leaf, Chickasawhay, and Bogue Chitto in Mississippi and eastern Louisiana. Louisiana ranks the Pascagoula map turtle as S3. In Louisiana, Dundee and Rossman (1989) considered populations of G. pulchra (now designated as G. gibbonsi) to be declining as a result of habitat degradation of the Pearl River system. These impacts include channelization for navigation and discharges of industrial effluent, particularly from the paper industry.
Escambia map turtle — Graptemys ernsti — G2
The Escambia map turtle was recently described as a new species, and like Graptemys gibbonsi, was previously considered to be a variant of G. pulchra (Lovich and McCoy, 1992). This species is ranked S2 in both Florida and Alabama and occurs in large to medium-sized river systems that empty into Pensacola Bay, including the Escambia, Conecuh, Yellow, and Shoal rivers of southern Alabama and western Florida. It is absent from streams that lack freshwater mollusks and is probably threatened by a variety of pollutants, including heavy metals, and by channelization (Florida Natural Areas Inventory, unpublished data). Shealey (1992) considered populations of G. pulchra (now designated as G. ernsti) to be rare in the Escambia and Yellow river drainages in Florida.
Ringed sawback turtle — Graptemys oculifera — G2
The ringed sawback turtle is federally listed as threatened. This species is ranked S2 and is state listed as endangered in Mississippi and ranked S2 in Louisiana. The decline in numbers of the ringed sawback turtle has been attributed to habitat modification and water quality deterioration in the Pearl and Bogue Chitto rivers, reservoir construction, channelization, de-snagging for navigation, siltation and subsequent loss of invertebrate food sources (S. Shively, Louisiana Natural Heritage Program, pers. comm.). On the Pearl River, fewer ringed sawback turtles were found downstream than upstream of Jackson, Mississippi, a major industrial and urban area. Poorer downstream water quality, which may impact the prey base, was suggested as the cause (Jones and Hartfield, 1995). Cagle (1953) found that ringed sawback turtles specialized and fed on aquatic insects that were associated with log snags.
Yellow-blotched sawback turtle — Graptemys flavimaculata — G2
The yellow-blotched sawback turtle is federally listed as threatened. It is endemic to the Pascagoula River system in Mississippi where it is ranked S2 and is state listed as endangered. Threats to this species are similar to those of the other Graptemys spp. and include profiteering in the pet trade, shooting of basking turtles, pollution, removal of snags, and channelization. In addition, high nest mortality as a result of increased flooding and inundation of sandbars and predation by fish crows (Corvus ossifragous), as well as low fecundity have been reported (LaClaire, 1995; Seigel and Brauman, 1995).
Black-knobbed sawback turtle — Graptemys nigrinoda — G3
The black-knobbed sawback turtle (Graptemys nigrinoda nigrinoda) is ranked S2 and is state listed as endangered in Mississippi and is ranked S3 in Alabama. Collection for the pet trade, shooting of basking turtles, the elimination of snags and sandbars, and channelization in the Tenn-Tom Waterway of the Tombigbee River are the probable causes related to the apparent decline in numbers (T. Mann, Mississippi Natural Heritage Program, pers. comm.). The delta map turtle (G. n. delticola) is ranked S2 in Alabama, where it is restricted primarily to the delta of the Mobile and Tensaw river systems (Conant and Collins, 1991).
Diamondback terrapin — Malaclemys terrapin — G5
The northern diamondback terrapin (Malaclemys terrapin terrapin), which ranges from Massachusetts to North Carolina, was formerly listed as C2 by the USFWS, and is ranked S3 and listed as special concern in North Carolina. Its status is undetermined in Virginia (Mitchell, 1991). North Carolina ranks the Carolina diamondback terrapin (M. t. centrata) as S3 and lists it as special concern. The mangrove diamondback terrapin (M. t. rhizophorarum) is ranked S2 in Florida and is restricted to mangrove habitats in the Florida Keys. It has been eliminated on most islands linked by U.S. Highway 1. As of 1982 it was still common around stands of black mangroves (Wood, 1992). The Mississippi diamondback terrapin (M. t. pileata) was formerly listed as C2 by the USFWS. Alabama ranks it as S2 and state protected and Louisiana ranks is as S2. The Texas diamondback terrapin (M. t. littoralis) occurs in Louisiana and Texas and was formerly listed as C2 by the USFWS. General degradation of salt marsh habitats, road associated mortality of nesting females, and drownings associated with crab pots are likely causes of population declines in all diamondback terrapin sub-species (Seigel and Gibbons, 1995).
Bog turtle — Clemmys muhlenbergii — G3
The USFWS formerly listed the southern populations of the bog turtle as C2. This secretive turtle occurs in mountain meadows and bogs of the southern Blue Ridge in Virginia, Tennessee, North Carolina, South Carolina, and Georgia. The largest populations exist in North Carolina and Virginia, where the turtle is ranked S2 and is state listed as threatened (North Carolina) and S1 and state listed as endangered (Virginia). The draining of wetlands, ditching of meadows, overgrazing, prevention of beaver activity, development, illegal collecting for the pet trade, and lack of environmental education among landowners and law enforcement personnel are cumulatively contributing to the decline of these turtles. Opportunities for metapopulation management and protection exist in both Virginia and North Carolina (Herman, 1988; Buhlmann et al., in press). In South Carolina, where the bog turtle is known from only two sites, which consist of impounded streams and beaver ponds, the turtle is ranked S1 and is state listed as threatened. Surveys of several classic montane bogs have failed to discover any specimens (S. Bennett, South Carolina Natural Heritage Trust, pers. comm.). Succession and tree canopy closure pose threats to bog turtle populations. Georgia ranks the bog turtle as S1 and lists it as threatened. The species has been reported from only three sites within Georgia, but survey work is ongoing (D. Herman, Zoo Atlanta, pers. comm.). Protection is currently being sought for the two known sites in Tennessee (B. Tryon, Knoxville Zoo, pers. comm.) where the bog turtle is ranked S1 and state listed as threatened.
Spotted turtle — Clemmys guttata — G5
Although not generally recognized as a species in decline, we suggest that populations of spotted turtles are imperiled. In the southeastern United States, spotted turtles inhabit blackwater cypress swamps, coastal plain wetlands, sinkhole ponds, and pine flatwoods ponds. South Carolina considers the spotted turtle as a species of concern (South Carolina Heritage Trust, 1996). Georgia ranks the spotted turtle as S3S4 (Georgia Natural Heritage Program, 1996). Populations in Florida (S3) are found in shallow woodland pools in wet pine flatwoods and are often disjunct from other populations. The species is suffering from population declines in the Coastal Plain of Virginia because of urban development, the destruction of wetlands in association with logging operations, and wetland drainage for agriculture. Martof et al. (1980) considered this turtle particularly vulnerable to habitat disruption resulting from drainage and development. The International Union for the Conservation of Nature (IUCN) recognizes the spotted turtle as a species in need of regulation due to the large volume of individuals involved in the pet trade. Unfortunately, spotted turtles can still be collected legally throughout much of their range. In North Carolina, 543 were known to have been recently removed in a single year (A. Braswell, North Carolina State Museum of Natural History, pers. comm.). If that many are known to have been removed, then certainly some multiple of that number was actually taken. Ditmars (1936) described this turtle as abundant.
Chicken turtle — Deirochelys reticularia — G5
The western chicken turtle (Deirochelys reticularia miaria) is ranked S2 in Arkansas and has been declining in numbers due to loss of shallow weedy ponds and swamps (Arkansas Natural Heritage Commission, unpublished data). The western chicken turtle is also found in cypress-bordered ponds in southeast Missouri where it is ranked S1 and is state listed as endangered. The turtle had not been verified in Missouri since 1962, until several individuals were found during 1995 field surveys (Buhlmann and Johnson, 1995). The floodplain swamps in the Missouri Bootheel region, where these turtles historically occurred, have been almost completely destroyed for agriculture. The eastern chicken turtle (D. r. reticularia) is ranked S1 and is state listed as endangered in Virginia. There, one disjunct population exists at the northern periphery of the species’ range in interdunal cypress ponds in a state park and natural area where no opportunities exist for immigration or emigration (Buhlmann, 1995). North Carolina ranks the eastern chicken turtle as S3 and considers it to be significantly rare (LeGrand and Hall, 1995). Chicken turtles are locally abundant in Carolina bays on the Savannah River Site in South Carolina (Buhlmann and Gibbons, authors’ unpublished data). Martof (1956) considered the eastern chicken turtle common in Georgia. In general, terrestrial movement is common in this species and road associated mortality is high.
Painted turtle — Chrysemys picta — G5
The southern painted turtle (Chrysemys picta dorsalis) is ranked S3 and listed as special concern in Kentucky. It is threatened by wetlands modification at the northern periphery of its range (Kentucky Nature Preserves Commission, unpublished data). It is also in a peripheral portion of its northern range in adjacent southeast Missouri, but appears secure at present (T. Johnson, Missouri Department of Conservation, pers. comm.).
River cooter — Pseudemys concinna — G5
The Suwannee cooter (Pseudemys concinna suwanniensis) is ranked S3 in Florida. Densities of these river turtles are presently less than they were historically (D. Jackson, Florida Natural Areas Inventory, pers. comm.). Major rivers in which this subspecies occurs have been degraded by dredging, impoundment, mining, and other sources of pollution. Large numbers of these turtles were once harvested for human consumption (Jackson, 1992). Carr (1940) reported that in the 1930s enormous aggregations of Suwannee cooters foraged on the flats off the mouth of the Suwannee River. Recently, Seidel (1994) proposed that P. c. suwanniensis be elevated to specific status, although this designation has been questioned by Jackson (1995). The status of the hieroglyphic cooter (P. c. hieroglyphica) is undetermined in Virginia, where it is recorded only from the Holston River in the extreme southwestern portion of the state (Mitchell, 1991).
Alabama redbellied turtle — Pseudemys alabamensis — G1
This turtle is an Alabama endemic ranked S1, state listed as protected, and federally listed as endangered. It is restricted to the lower Mobile Bay and tributary streams in Mobile and Baldwin counties (Ernst et al., 1994). It reaches its greatest abundance in the uppermost portion of Mobile Bay in fresh to moderately brackish water that supports an abundance of submergent vegetation (Mount, 1975). The numbers of juvenile Alabama redbellied turtles apparently declined between 1970 and 1983 (Dobie and Bagley, 1990). Nesting was previously thought to be confined to one island, but is now known to occur on several spoil banks and along a causeway in Mobile Bay (D. Nelson, University of South Alabama, pers. comm.). Development, pollution, collecting for the pet trade, and a limited range are cited as causes for concern (Ernst et al., 1994).
Florida redbellied turtle — Pseudemys nelsoni — G5
Relatively abundant in Florida, the Florida redbellied turtle reaches the northern edge of its range in the Okefenokee Swamp in southeastern Georgia where it is ranked S2.
Mississippi redbellied turtle — Pseudemys sp. — G1
This undescribed redbellied turtle was formerly listed as C2 by the USFWS. It is a Mississippi endemic that is restricted to the lower Pascagoula River (J. Dobie, Auburn University, unpublished data). It is ranked S1 and state listed as endangered in Mississippi. Threats to this turtle likely include those mentioned previously for Graptemys flavimaculata. Prior to 1950, shrimp trawlers reported capturing large numbers of "cooters" in the Mississippi Sound and this species may have been formerly abundant there (Mississippi Department of Wildlife, Fisheries, and Parks, 1992). All-terrain vehicles have been implicated as responsible for the disturbance of this turtle’s nesting beaches. Morphological differences suggest that the Mississippi red-bellied turtle is unique from the Alabama red-bellied turtle, but the biochemical genetic differences are currently unresolved (C. Lydeard, University of Alabama, pers. comm.).
Slider turtle — Trachemys scripta — G5
In Virginia, the Cumberland slider (Trachemys scripta troosti) exists at the northeastern margin of its range (Mitchell, 1994) and is ranked S1. In Virginia it is known only from the Holston River and probably faces the same water pollution threats as already described for Sternotherus minor peltifer and Graptemys geographica. Tennessee ranks the Cumberland slider as S3S4. In Louisiana, at least 25 turtle farms have been reported to remove 100,000 mature individuals of the red-eared slider (T. s. elegans) from the wild annually (Warwick, 1986; Warwick et al., 1990). Data support the hypothesis that harvesting of adult sliders is having a significant impact on turtle populations in southern Louisiana. Turtles from harvested sites were significantly smaller in body size than turtles from protected sites (Close and Seigel, in press). The International Union for the Conservation of Nature is considering regulations restricting trade in this species, as large numbers are exported annually to Asian markets for food and European countries as pets. Populations of red-eared sliders have become established outside of their native range due to pet releases (Moll et al., in press). Many populations of yellow bellied sliders (T. s. scripta) from southeastern Virginia display morphological characteristics that indicate genetic influence from T. s. elegans (Mitchell, 1994).
Florida softshell turtle — Trionyx (Apalone) ferox — G5
The Florida softshell turtle reaches its northernmost range in South Carolina in ponds, lakes, and rivers of the Combahee and Savannah river system (Martof et al., 1980). This species is not well known in South Carolina and has been ranked SU and listed as special concern. At the western edge of its range in Alabama, it is ranked S2 and is state listed as protected. This softshell inhabits lakes, big clear water springs, permanent ponds, and occasionally slow-moving stretches of rivers (Conant and Collins, 1991). Recent information suggests that Florida softshell turtles are being harvested in Florida and shipped to Japan for food (Ft. Pierce Tribune, 1996).
Smooth softshell turtle — Trionyx (Apalone) mutica — G5
In Florida, the Gulf Coast smooth softshell turtle (Trionyx mutica calvata) is ranked S2 and has a limited distribution that includes the Escambia River. This subspecies also occurs in the Pearl and Tombigbee rivers in Mississippi, two parishes in Louisiana (ranked S3), and in the Conecuh, Escambia, and Mobile Bay drainages in Alabama. Although the species has been poorly surveyed, habitat degradation in the big rivers may be a concern (S. Shively, Louisiana Natural Heritage Program, pers. comm.). The midland smooth softshell turtle (T. m. mutica) is found in the Ohio River in Kentucky where riverine water quality might be a concern and where it is ranked S3 and listed as special concern. In a study of both T. mutica and spiny softshell turtles (T. spinifera), Doody (1995) found that flooding of sandbars resulted in nest mortality. These results suggest that increases in flooding as a result of channelization may contribute to population declines. Doody (1995) also found that 21 percent of all predated nests were destroyed by introduced fire ants (Solenopsis sp.).
Spiny softshell turtle — Trionyx (Apalone) spinifera — G5
The eastern spiny softshell turtle (Trionyx spinifera spinifera) is ranked S2 in Virginia, and it is ranked S1 and is listed as special concern in North Carolina. In those states, it inhabits rivers of the Tennessee River drainage, specifically the Clinch, Holston, Powell, and French Broad rivers, and Copper Creek. Threats to this species include poor water quality in rivers and are generally the same as those described previously for Sternotherus minor peltifer and Graptemys geographica. The Gulf Coast spiny softshell turtle (T. s. aspera) is ranked S3 in North Carolina where it reaches the northern edge of its range in southcentral river systems.
Worldwide, the great majority of documented reptilian extinctions directly attributable to human-related disturbance have been on islands (Case et al., 1992). A minimum of 60 Holocene (Recent) reptile extinctions (13 percent on continents) are known to have occurred, compared to 115 extinctions of mammals (64 percent on continents), and 171 extinctions of birds (ten percent on continents). On continents, reptiles and birds have fared better than mammals, but birds have suffered more than either of the other groups on islands, judged on the basis of documented occurrences and disappearances. Overall, reptiles appear to have been slightly more resilient to human disturbance than birds or mammals (Case et al., 1992). For example, in striking contrast to the severely depleted mammalian megafauna, only three reptilian extinctions in North America (tortoise, Geochelone wilsonii; horned lizard, Phrynosoma josecitensis; and a large rattlesnake, Crotalus potterensis) are known from the approximately 130 fossil species described from the continental United States since the Pleistocene (about 10,000 years before the present) (Moodie and Van Devender, 1979). All of these reptiles were terrestrial, and the disappearance of none has been confirmed to have been in response to human environmental impacts.
However, many reptile species are clearly not faring well today. For example, Lydeard and Mayden (1995) considered 43 percent of Alabama’s freshwater turtles to be imperiled. Lovich (1995) considered 45 percent of the total United States turtle species to require conservation action. We consider 62 percent of the total aquatic reptile fauna in the southeastern United States to be at some risk of extinction or significantly declining in numbers in at least a portion of their range (Table 3).
In this review we have included taxa that are rare or threatened due to their peripheral occurrences in one or more of the states in the Southeast. Generally, most of these taxa have wide distributions, occur in a variety of habitat types, and are not threatened globally. For example, in Kentucky, the broad-banded water snake (Nerodia fasciata confluens) and the southern painted turtle (Chrysemys picta dorsalis) are threatened by wetlands modification. Both taxa currently appear secure further south. In Georgia, the striped crayfish snake (Regina alleni), and the Florida redbellied turtle (Pseudemys nelsoni) reach their northernmost distributions in the Okefenokee Swamp and are considered rare due to their limited state distribution. Both taxa seem to be common in Florida. At the northern extent of its range, in extreme southeastern Virginia, the eastern glass lizard (Ophisaurus ventralis) occurs only in freshwater wetlands between coastal dunes (Mitchell, 1994). Eastern glass lizards occur in a wide range of habitats further south.
The identification and protection of taxa at the fringes of their ranges are important for conservation. Populations of species that exist at the edge of their ranges are likely to exhibit genetic differences from populations at the center because different selection pressures may be acting on them. These populations may already be physiologically stressed and therefore susceptible to human disturbance. Conversely, natural selection may function most strongly on fringe populations and hence these populations may have great importance for the long-term continuance of evolutionary processes. The rate of evolutionary change in a population is proportional to the amount of genetic diversity available (Fisher, 1930). Therefore, the reduction of intraspecific genetic diversity, as possibly caused by the extinction of peripheral or fringe populations, may limit the future ability of said species to survive under changing environmental conditions (Meffe and Carroll, 1994).
The decline of the alligator snapping turtle (Macroclemys temminckii) is a direct result of unregulated or poorly regulated harvest (Sloan and Lovich, 1995). Congdon et al. (1994) have shown that populations of common snapping turtles (Chelydra serpentina) cannot withstand continued cropping of adults. Removal of long-lived, slow-growing animals with life history strategies aimed at replacement reproduction spread out over the lifetimes of the animals will cause a population decline (Congdon et al., 1993). Based on the reports of continued harvest and continued degradation of riverine systems, the future looks grim for alligator snapping turtles. Other turtles, primarily Graptemys ssp., Clemmys guttata, and Clemmys muhlenbergii have been exploited for the pet trade. Removal of individuals from populations that are already reduced because of human degradation of habitat represents another additive impact on populations. Even presently abundant species (e.g., Trachemys scripta elegans) should be considered of concern in some instances because of the vast numbers being removed from the wild and shipped to other countries.
Table 3. Numbers of aquatic reptile taxa (all recognized species and subspecies) occurring within the southeastern United States that fall into imperiled or unimperiled categories. Imperiled taxa are defined as taxa of conservation concern in at least one southeastern state within which they occur. Percent of total taxa reported in parentheses. |
|||
Group |
Total Taxa |
Imperiled Taxa |
Presumed Unimperiled |
Crocodilians |
2 |
2 (100) |
0 (0) |
Lizards |
2 |
2 (100) |
0 (0) |
Snakes |
39 |
22 (56.4) |
17 (43.6) |
Turtles |
57 |
36 (63.2) |
21 (36.8) |
Totals |
100 |
62 (62) |
38 (38) |
Destruction and alteration of wetland habitats, which include Carolina bays, cypress swamps, bottomland hardwoods, pine flatwoods ponds, mountain bogs, and salt marshes, have resulted in population declines of 34 aquatic snake and turtle taxa. The fragmentation and isolation of remaining wetlands subject populations to a greater risk of extinction through the loss of immigration and emigration opportunities, inbreeding, and random environmental perturbations.
Taxa at greatest risk from wetland habitat losses include the green water snakes (Nerodia cyclopion and N. floridana), copperbellied watersnake (N. erythrogaster neglecta), swamp snake (Seminatrix pygaea), rainbow snake (Farancia erythrogramma), and the glossy crayfish snake (Regina rigida rigida). Turtles at risk for the same reasons include the spotted turtle (Clemmys guttata), bog turtle (C. muhlenbergii), and chicken turtle (Deirochelys reticularia). The alteration, development, and pollution of salt marsh and mangrove swamp habitats have been shown to be the primary causes of declines in the salt marsh snakes Nerodia clarkii and N. sipedon williamengelsi, diamondback terrapins (Malaclemys terrapin), and American crocodile (Crocodylus acutus).
Overall, 22 southeastern reptile taxa (20 turtles, two snakes) show declines attributable to river and stream degradation. Among the most imperiled are species endemic to single or a few river systems. The map turtles (Graptemys spp.) are characterized by great taxonomic diversity and drainage-specific endemism, primarily Coastal Plain drainages along the Gulf of Mexico coast from the Appalachicola River in Florida to the Guadalupe River in Texas (Lovich and McCoy, 1992). Seven of the 13 species are restricted to single drainage systems.
Pollution, channelization, shooting, harvesting, and disease are all causes for declines of map turtles. In general, some of the musk turtles (Sternotherus spp.), the alligator snapping turtle (Macroclemys temminckii), and several Pseudemys spp. are imperiled for the same reasons. The documented declines in riverine fish faunas often parallel declines in riverine reptile faunas. Declines in fish species in large polluted rivers have often been attributed to chronic, sub-lethal pollution, which cause fish to disappear gradually as they produce fewer young, grow more slowly, and die from stress-related diseases (Moyle and Leidy, 1992). Many river fishes assimilate heavy metals and pesticides in their tissues. Aquatic turtles with relatively longer lifespans have been poorly studied with regard to chronic exposure to pollutants.
Sheldon (1988) stated that the best protection strategy for rivers would be to focus conservation efforts on the largest tributaries that contain their original faunas in as many regions as possible. In our assessment, river-dwelling reptiles are at the highest level of imperiled status and should be considered a high conservation priority.
The opportunity for populations to exchange genetic material and find appropriate habitat when their current habitat becomes unsuitable is critical for their long-term persistence. Alteration of terrestrial habitats due to clearcutting, farming, highway construction, and other forms of development has left many wetland habitats isolated in a surrounding hostile landscape matrix. Riverine habitats are subject to fragmentation because reservoirs inhibit or stop the movement of individuals and isolate riffle habitats between deep lake habitats.
Populations of organisms that live in wetland habitats must be able to migrate to other wetland locations when their current habitats become unsuitable due to natural causes such as succession and drought. For example, slider turtle (Trachemys scripta) data showed that during a drought starting in 1985, most individuals exited Ellenton Bay, a typical Carolina bay habitat on the SRS, and moved toward water in a nearby beaver pond. When the drought ended, the turtles began to return (Burke et al., 1995).
During the drying period of 1986 through 1988 at Ellenton Bay, more than 189 snakes of three species were captured at the drift fence as they departed the area (Seigel et al., 1995). Banded water snakes (Nerodia fasciata) apparently left the site in direct response to low water levels, whereas swamp snakes (Seminatrix pygaea) left due to the absence of small fish (Gambusia sp.) and salamander (Ambystoma sp.) prey. Few green water snakes (Nerodia floridana) were recorded leaving, and the snakes that remained presumably perished. However, all species are again present at this site. The green water snake, unobserved at Ellenton Bay for five years post-drought, was first seen again in the spring of 1993, but numbers are less than pre-drought.
Turtles originally marked in Ellenton Bay were captured in a variety of more permanent aquatic sites during the next few years after the aforementioned period of drought, some up to 3.1 miles (5 km) away. If the neighboring aquatic habitats had been unavailable for these turtles to inhabit throughout the period of drought, the Ellenton Bay population would perhaps have been completely eliminated in response to this natural climatic event. Although we have no data on the exact dispersal patterns of snakes, we assume that many also weathered the drought by emigrating from Ellenton Bay to find temporary sanctuaries in nearby bodies of permanent water.
Many aquatic reptiles require the adjacent terrestrial habitat at certain seasons. For example, the exact locations of nests and terrestrial hibernation sites of mud turtles (Kinosternon subrubrum) were recorded by Burke and Gibbons (1995) in South Carolina. All locations were outside the federal wetland delineation line. Likewise, most individuals in a nearby population of chicken turtles (Deirochelys reticularia) wintered in forested upland habitats 164-820 feet (50-250 m) from the wetland delineation margin of a Carolina bay (K. A. Buhlmann and J. W. Gibbons, authors’ unpublished data). Understanding the natural history, including specific habitat needs, of wetland species is crucial for effective conservation planning (Buhlmann et. al., 1993a).
The diamondback terrapin (Malaclemys terrapin) serves as an example of the resilience of at least one native species to what humans regard as a severe natural disaster. In 1990, Hurricane Hugo hit the southeast coast around Charleston, South Carolina. Its commercial impact was catastrophic, yet its impact on terrapins was inconsequential. Until 1990, researchers at Savannah River Ecology Lab had captured and marked more than 500 terrapins in the tidal creeks and marshes around Kiawah Island, close to the focal point of the storm. Post-hurricane recaptures suggest that the diamondback terrapins in this healthy population were unaffected on a population level (J. W. Gibbons, author’s unpublished data). This may not have been the case if the terrapins were already declining in numbers due to destruction of the marsh habitat and removal for commercial purposes.
Regrettably, we know so little about many species of aquatic reptiles that we can only speculate on the best ways to provide protection. Reptiles are often secretive creatures that require special capture techniques. Simply documenting their presence is not always easy. The SRS in South Carolina has been one of the most intensively studied tracts of land for reptiles in North America and gives some insight into this problem. More than 50,000 reptiles of 57 species have been captured during the past 41 years, and the species list is still growing. Freeman (1955) recorded 44 species; Duever (1967) 49; Gibbons (1977) 50; Gibbons and Patterson (1978) 51; and Gibbons and Semlitsch (1991) 57. Had aquatic habitats on the SRS been degraded or destroyed rather than protected since the 1950s, the presence of many of these species would probably have gone unrecognized.
Simply documenting a species’ presence is only the first step toward ecological understanding. State and federal agencies should fund basic research including an inventory of the nation’s biodiversity. It is also the responsibility of educators at the university level to explain the value of protecting biodiversity to the general public and to politicians.
Turtles are declining in both number of species and total numbers at alarming rates throughout the world (Ernst and Barbour, 1989). Worldwide, losses have been attributed to causes that include the collecting of eggs, juveniles, and adults for the pet trade and human food consumption. Long-lived species with delayed sexual maturity and low reproductive rates cannot sustain continued high levels of harvest and maintain stable populations. Destruction and deterioration of natural habitats has led to reduced population sizes and to the fragmentation and isolation of remaining populations. For reptiles, the ever-increasing web of roads that spans the landscape serves to increase the probability of mortality for individuals of many species, leading to eventual local population reductions or extinctions.
We found that 35.5 percent of our list of imperiled aquatic reptiles are threatened because of the continuing, cumulative abuse sustained by river systems. Another 37.1 percent are declining due to loss of Coastal Plain wetland habitats, 1.6 percent due to loss of mountain wetlands, 13.0 percent due to losses of brackish and salt marshes, and 3.2 percent due to loss of prairie wetlands.
If we are serious about protecting biodiversity and halting wildlife population declines in the Southeast, several intellectually simple, yet politically difficult changes must be made. First, the destruction and alteration of remaining wetlands must cease. The idea that landowners have the right to do whatever they wish to the natural resources on their land needs to be re-thought and openly addressed. Secondly, river environments must be restored and protected. Our river-dwelling reptile fauna is the most immediately imperiled reptile group. The cumulative effects of industrial wastes, pesticides, herbicides, oil runoff, inappropriate and careless farming practices, and other environmental insults could ultimately relegate the rivers to little more than shipping canals. The cumulative effects of this abuse have resulted not only in declines of aquatic fauna but also present a real threat to human health as well. Downstream of almost every effluent discharge point is a drinking water intake. If people truly understood the dangers they face from the current treatment of our rivers, major changes could be made. Lastly, and possibly most important, human population growth must be controlled. The human population cannot keep growing exponentially (Meffe et al., 1993). People require space and resources that displace wildlife. The population problem is a concern that most choose to ignore, but it is the rooted cause of most, if not all, of our environmental problems.
The following state biologists were invaluable in providing expertise and information about southeastern aquatic reptiles: Cindy Osborne, Arkansas Natural Heritage Commission; Steve Bennett, South Carolina Department of Natural Resources; David J. Printiss and Dale R. Jackson, both Florida Natural Areas Inventory; Tom Mann, Mississippi Natural Heritage Program; Greg Krakow, Georgia Natural Heritage Program; David I. Withers, Tennessee Ecological Services Division; Tom Bloom, Kentucky State Nature Preserves Commission; Megan G. Rollins, Steven M. Roble, and Christopher H. Hobson, all Virginia Division of Natural Heritage; Tom R. Johnson, Missouri Department of Conservation; Harry LeGrand, North Carolina Natural Heritage Program; Mark Bailey, Alabama Natural Heritage Program; Steve Shively, Louisiana Natural Heritage Program; and Melissa Morrison, TNC, Eastern Heritage Task Force. The manuscript was improved with comments from J. C. Mitchell, R. A. Seigel, D. Collins, G. W. Benz, S. McKeon and M. Plummer. Funding for manuscript preparation was provided by contract number DE-AC09-76SR00-819 between the U.S. Department of Energy and the University of Georgia’s Savannah River Ecology Laboratory.