
Aquatic mollusks in the southeastern United States reside in a wide variety of permanent and seasonal aquatic habitats, and the diversity of bivalves and gastropods in the Southeast is globally unparalleled. Aquatic mollusks are distributed throughout the many tributaries of major rivers in the Interior Basin that drain various physiographic provinces along the South Atlantic and Gulf Coasts. Rivers of the southern Interior Basin, and of the Coastal Plain, originate in or traverse through several physiographic provinces: Appalachian Highlands, Ridge and Valley, Blue Ridge, Piedmont Plateau, Cumberland Plateau, and Coastal Plain. Regional and historical differences in physiography, geology, water chemistry, and other stream characteristics have resulted in distinct faunal assemblages and considerable endemism within river basins. Heard (1970) attributed striking differences in the southeastern freshwater molluscan fauna to assemblages adapted to specific lotic conditions (small streams vs. large rivers) and to locales with differences in specific environmental conditions (substratum, food availability, etc.). Riverine ecosystems may account for the highest diversity of freshwater mollusks because they are more permanent in an evolutionary time scale than lakes or other freshwater environments. They also contain a greater heterogeneity of physico-chemical characteristics and biological niches for aquatic organisms to adapt to and evolve with — from small headwater streams with swift current and allochthonous energy contributions to large coastal plain rivers with slow flow and autochthonous production.
A plethora of natural and anthropogenic factors have influenced the current distribution of freshwater mollusk species. However, differentiating between these factors is difficult without sufficient historic surveys and collection records to support conclusions. Extensive biological inventories were never conducted in most Southeast aquatic ecosystems. Therefore, the degree of aquatic faunal losses is unknown (Schindler, 1989). Because freshwater mollusks have never been a faunal group of great interest to federal and state natural resource agencies, much historic knowledge is derived from the collections and writings of early naturalists who traveled the eastern United States, in search of new animals and environments. Unfortunately, the early taxonomy and systematics of freshwater mollusks were based principally on shell characteristics that vary within and between rivers. As a result, an abundance of nominal species was described in the 19th century (Rafinesque, 1820, 1831; Lea, 1834-1874). Only in the 20th century has a concerted effort been made to evaluate early descriptions, identify synonomies, and compile a more accurate list of resident mollusk species in the rivers of the United States. For purposes of this chapter, we adopt the nomenclature of Turgeon et al. (1988).
Species richness of freshwater mollusks in the United States consists of more than 850 species in three taxonomic groups (Table 1). Snails are the most diverse taxon, accounting for 60 percent of all mollusk species. When this species richness is assessed from a regional perspective, it is readily apparent that the "rain forest" of mollusk diversity is in the southeastern United States. Based on Turgeon et al. (1988) and taxonomic keys and distribution records, we calculate that 91 percent of the mussels, 53 percent of the fingernail clams, and 61 percent of the snails in the United States occur in one or more states of the Southeast (Table 1). It is important to acknowledge that new species of freshwater mollusks continue to be described (Thompson and Hershler, 1991; Bogan and Hoeh, 1994), because sampling in localized habitats and new genetic techniques provide more decisive data and tools to help indicate the phylogenies and origins of mollusks in the Southeast. These tallies of species richness, especially for the mussels and snails, will undoubtedly increase.
Table 1. Species richness of freshwater mollusks in the southeastern United States. |
||
Taxonomic Group |
Number of U.S. Species |
Number of Species in Southeast 1 |
Mussels |
297 |
269 (91) |
Fingernail Clams |
38 |
20 (53) |
Snails |
516 |
313 (61) |
1 Percent of U.S. species in parentheses. |
||
Much knowledge of affinities among mollusk assemblages in rivers is derived
from studies of freshwater mussels (Unionoidea). Unionids offer three advantages
for 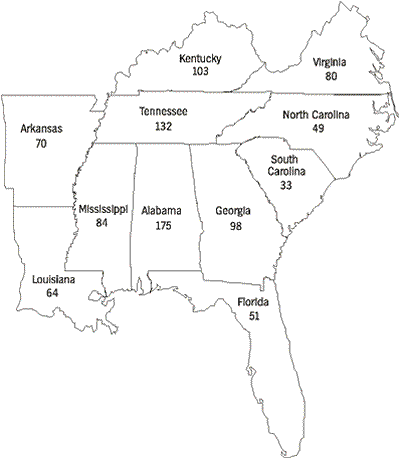 zoogeographic
study: they are relatively sedentary, reasonable numbers of species are readily
distinguishable, and generic affinities have been fairly well defined (Burch,
1973). The dispersal ability of mussels is restricted principally to the glochidial
stage and the mobility of their host fish species. Freshwater fishes are generally
confined to specific river drainages and can migrate between adjacent river
basins only after physiographic changes to the landscape, such as stream captures
or base leveling during glacial events. For these reasons, mussel distributions
seem to be excellent indicators of physiographic change between adjacent river
systems through geologic time. Molluscan faunal zones have been defined by their
distinctive mussel and snail assemblages — sharing various numbers of species
with other rivers according to drainage modification, isolation, confluence,
stream capture, and other phenomena from regional or global events such as glaciation
and sea level changes. The direct and indirect effects of these events on local
biota and ecology have shaped distinctive communities with traceable phylogenies.
For example, mussels have provided convincing evidence of major stream confluences
(van der Schalie, 1945). Historic connections between the Apalachicola and Savannah
rivers are suggested by their mollusk assemblages. Suffice it to say that lengthy
discussions of zoogeography and zonation of aquatic fauna in the Southeast have
been made possible by the distribution patterns of mollusks (van der Schalie
and van der Schalie, 1950; Johnson, 1970, 1980; Hocutt and Wiley, 1986).
zoogeographic
study: they are relatively sedentary, reasonable numbers of species are readily
distinguishable, and generic affinities have been fairly well defined (Burch,
1973). The dispersal ability of mussels is restricted principally to the glochidial
stage and the mobility of their host fish species. Freshwater fishes are generally
confined to specific river drainages and can migrate between adjacent river
basins only after physiographic changes to the landscape, such as stream captures
or base leveling during glacial events. For these reasons, mussel distributions
seem to be excellent indicators of physiographic change between adjacent river
systems through geologic time. Molluscan faunal zones have been defined by their
distinctive mussel and snail assemblages — sharing various numbers of species
with other rivers according to drainage modification, isolation, confluence,
stream capture, and other phenomena from regional or global events such as glaciation
and sea level changes. The direct and indirect effects of these events on local
biota and ecology have shaped distinctive communities with traceable phylogenies.
For example, mussels have provided convincing evidence of major stream confluences
(van der Schalie, 1945). Historic connections between the Apalachicola and Savannah
rivers are suggested by their mollusk assemblages. Suffice it to say that lengthy
discussions of zoogeography and zonation of aquatic fauna in the Southeast have
been made possible by the distribution patterns of mollusks (van der Schalie
and van der Schalie, 1950; Johnson, 1970, 1980; Hocutt and Wiley, 1986).
Because few extensive or intensive historic surveys were conducted on southeastern freshwater mollusk taxa, except for perhaps freshwater mussels, it is not possible to document the many changes in diversity, abundance, and distribution that have occurred in the last 100 years. Therefore, we are unable to describe the extent of decline of many mollusk groups throughout the Southeast. Our approach here is to select river systems with historic and recent collection records to serve as case studies for mollusks, principally freshwater mussels and river snails. These are the most diverse families of mollusks in the Southeast, and they are suitable indicators of change to their communities and environments. Other families of bivalves (fingernail clams: Sphaeriidae) and gastropods (e.g., freshwater limpets: Ancylidae) have been too poorly surveyed or sampled to provide an assessment of species stability or decline. Hopefully the results of this paper will stimulate interest in determining the status of other families and genera of mollusks in this region. There is still much to be done in the taxonomy, biology, and ecology of freshwater mollusks so that appropriate conservation efforts can be directed to those taxa or habitats in need of protection or recovery.
Freshwater mussels of the families Unionidae and Margaritiferidae are the best studied group of freshwater mollusks in the United States, with adequate historic and recent collection records to document changes in distribution and abundance of many species. Of the 297 species and subspecies currently recognized (Turgeon et al., 1988), 269 species had historic ranges that overlapped the political boundaries of one or more states of the Southeast. Species richness varies among southeastern states, ranging from an historic high of 175 species in Alabama to 33 species in South Carolina (Figure 1). These totals were compiled principally during the early 20th century and have changed drastically during the last 70 years.
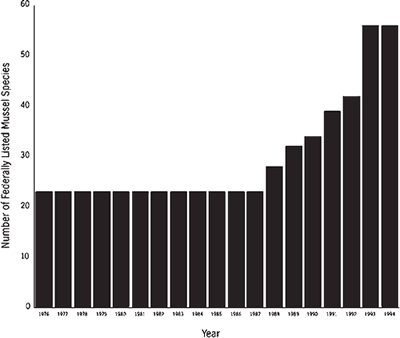
The Endangered Species Act of 1973 and subsequent amendments provided the legal means for recognition of rare mollusks that deserve federal protection. In June 1976, 23 species of freshwater mussels were designated as endangered. Because of internal priorities in the U.S. Fish and Wildlife Service and because of the overwhelming number of vertebrate and invertebrate species deserving of consideration under the Act, no additional species of mussels were listed until 1988 (Figure 2). Since then, a profound increase in listings has reflected the recognition of serious declines in freshwater bivalves by field biologists of the U.S. Fish and Wildlife Service, particularly in the Southeast. As of January 1995, 56 mussel species are federally listed as endangered or threatened in the United States. Except for the Curtis pearlymussel (Epioblasma florentina curtisi) in Missouri and the white catspaw (E. obliquata perobliqua) in Indiana, Michigan, and Ohio, the 53 other listed species were known historically from one or more states in the Southeast (Table 2). In addition to these protected species, federal biologists identified 51 candidate species of mussels awaiting evaluation for possible listing (Table 3), all of which occur in southeastern states. Thus, more than 34 percent of all mussel species nationwide are in varying degrees of rarity, and 98 percent of these rare species occur in the Southeast.
Table 2. Species of freshwater mussels and snails federally designated as endangered or threatened in the southeastern United States in 1994. |
|||
Scientific Name |
Common Name |
Historic Range |
Status |
Mussels: |
|||
|
dwarf wedgemussel |
CT, DC, DE, MA, MD, NC, NH, PA, VT, VA |
E |
|
Ouachita rock pocketbook |
AR, OK |
E |
|
fanshell |
AL, IL, IN, KY, OH, PA, TN, VA, WV |
E |
|
dromedary pearlymussel |
AL, KY, TN, VA |
E |
|
Tar spinymussel |
NC |
E |
|
yellow blossom |
AL, TN |
E |
|
tan riffleshell |
KY, TN, VA |
E |
|
upland combshell |
AL, GA, TN |
E |
|
catspaw |
AL, IL, IN, KY, OH, TN |
E |
|
southern acornshell |
AL, GA, TN |
E |
|
southern combshell |
AL, MS |
E |
|
green blossom |
TN, VA |
E |
|
northern riffleshell |
IL, IN, KY, MI, OH, PA, WV |
E |
|
tubercled blossom |
IL, IN, KY, TN, WV |
E |
|
turgid blossom |
AL, TN |
E |
|
shiny pigtoe |
AL, TN, VA |
E |
|
fine-rayed pigtoe |
AL, TN, VA |
E |
|
cracking pearlymussel |
AL, IL, IN, KY, OH, TN, VA |
E |
|
pink mucket |
AL, IL, IN, KY, MO, OH, PA, TN, VA, WV |
E |
|
fine-lined pocketbook |
AL, GA |
E |
|
orange-nacre mucket |
AL, MS |
T |
|
Arkansas fatmucket |
AR |
T |
|
speckled pocketbook |
AR |
E |
|
Alabama lampmussel |
AL, TN |
E |
|
Carolina heelsplitter |
NC, SC |
E |
|
birdwing pearlymussel |
AL, TN, VA |
E |
|
Louisiana pearlshell |
LA |
T |
|
Alabama moccasinshell |
AL, GA, MS |
T |
|
Coosa moccasinshell |
AL, GA, TN |
E |
|
ring pink |
AL, IL, IN, KY, OH, PA, TN, WV |
E |
|
little-wing pearlymussel |
AL, KY, NC, TN, VA |
E |
|
white wartyback |
AL, IN, TN |
E |
|
orange-foot pimpleback |
AL, IN, IA, KY, OH, PA, TN |
E |
|
clubshell |
AL, IL, IN, KY, MI, OH, PA, TN, WV |
E |
|
James spinymussel |
VA, WV |
E |
|
black clubshell |
AL, MS |
E |
|
southern clubshell |
AL, GA, MS, TN |
E |
|
dark pigtoe |
AL |
E |
|
southern pigtoe |
AL, GA, TN |
E |
|
Cumberland pigtoe |
TN |
E |
|
flat pigtoe |
AL, MS |
E |
|
ovate clubshell |
AL, GA, MS, TN |
E |
|
rough pigtoe |
AL, IN, KY, TN, VA |
E |
|
heavy pigtoe |
AL, MS |
E |
|
fat pocketbook |
AR, IN, MO, OH |
E |
|
inflated heelsplitter |
AL, LA, MS |
T |
|
triangular kidneyshell |
AL, GA, TN |
E |
|
winged mapleleaf |
IA, IL, IN, KY, MN, MO, NE, OH, OK, TN, WV |
E |
|
Cumberland monkeyface |
AL, TN, VA |
E |
|
Appalachian monkeyface |
AL, TN, VA |
E |
|
stirrupshell |
AL, MS |
E |
|
pale lilliput |
AL, TN |
E |
|
Cumberland bean |
KY, TN |
E |
Snails: |
|||
|
tulotoma snail |
AL |
E |
|
Anthony's riversnail |
AL, TN |
E |
|
royal marstonia |
TN |
E |
Table 3. Species of freshwater mussels on the federal candidate list in the southeastern United States in 1994.1 |
||
Scientific Name |
Common Name |
State(s) of Occurrence |
Alasmidonta arcula (Lea, 1838) |
Altamaha arc-mussel |
GA |
A. atropurpurea (Rafinesque, 1831) |
Cumberland elktoe |
KY, TN |
A. raveneliana (Lea, 1834) |
Appalachian elktoe |
NC |
A. varicosa (Lamarck, 1819) |
brook floater |
GA, NC, SC, VA |
A. wrightiana (Walker, 1901) |
Florida arc-mussel |
FL |
Amblema neislerii (Lea, 1858) |
fat three-ridge |
FL, GA |
Anodontoides denigrata (Lea, 1852) |
Cumberland papershell |
KY, TN |
Cumberlandia monodonta (Say, 1829) |
spectaclecase |
AL, AR, KY, TN, VA |
Cyprogenia aberti (Conrad, 1850) |
western fanshell |
AR |
Elliptio sp. |
Waccamaw lance |
NC |
E. chipolaensis Walker, 1905 |
Chipola slabshell |
AL, FL |
E. judithae Clarke, 1986 |
Neuse slabshell |
NC |
E. lanceolata (Lea, 1828) |
yellow lance |
NC, VA |
E. marsupiobesa Fuller, 1972 |
Cape Fear spike |
NC |
E. monroensis (Lea, 1843) |
St. Johns elephantear |
FL |
E. nigella (Lea, 1852) |
winged spike |
AL, GA |
E. shepardiana (Lea, 1834) |
Altamaha lance |
GA |
E. spinosa (Lea, 1836) |
Altamaha spinymussel |
GA |
E. waccamawensis (Lea, 1863) |
Waccamaw spike |
NC |
E. waltoni (Wright, 1888) |
Florida lance |
FL |
Elliptoideus sloatianus (Lea, 1840) |
purple bankclimber |
AL, GA, FL |
Epioblasma brevidens (Lea, 1831) |
Cumberlandian combshell |
AL, KY, TN, VA |
E. capsaeformis (Lea, 1834) |
oyster mussel |
AL, KY, TN, VA |
E. triquetra (Rafinesque, 1820) |
snuffbox mussel |
AL, KY, MS, TN, VA |
Fusconaia escambia Clench and Turner, 1956 |
narrow pigtoe |
AL, FL |
F. masoni (Conrad, 1834) |
Atlantic pigtoe |
GA, NC, SC, VA |
Lampsilis australis Simpson, 1900 |
southern sandshell |
AL, FL |
L. binominata Simpson, 1900 |
lined pocketbook |
AL, GA |
L. cariosa (Say, 1817) |
yellow lampmussel |
GA, NC, SC, VA |
L. fullerkati Johnson, 1984 |
Waccamaw fatmucket |
NC |
L. rafinesqueana Frierson, 1927 |
Neosho mucket |
AR |
L. subangulata (Lea, 1840) |
shiny-rayed pocketbook |
AL, FL, GA |
Lasmigona sp. |
Barrens heelsplitter |
KY |
L. holstonia (Lea, 1838) |
Tennessee heelsplitter |
AL, GA, KY, TN, VA |
L. subviridis (Conrad, 1835) |
green floater |
NC, SC, VA |
Leptodea leptodon (Rafinesque, 1820) |
scaleshell |
AR, KY |
Lexingtonia dolabelloides (Lea, 1840) |
slabside pearlymussel |
AL, TN, VA |
Margaritifera marrianae Johnson, 1983 |
Alabama pearlshell |
AL |
Medionidus penicillatus (Lea, 1857) |
Gulf moccasinshell |
AL, FL, GA |
M. simpsonianus Walker, 1905 |
Ochlocknee moccasinshell |
FL |
M. walkeri (Wright, 1897) |
Suwanee moccasinshell |
FL |
Obovaria rotulata (Wright, 1899) |
round ebonyshell |
AL, FL |
Pleurobema oviforme (Conrad, 1894) |
Tennessee clubshell |
KY, TN, VA |
P. pyriforme (Lea, 1857) |
oval pigtoe |
AL, FL, GA |
P. rubellum (Conrad, 1834) |
Warrior pigtoe |
AL |
P. rubrum (Rafinesque, 1820) |
pink pigtoe |
AL, KY, MS, TN, VA |
P. strodeanum (Wright, 1898) |
fuzzy pigtoe |
AL, FL |
P. verum (Lea, 1860) |
true pigtoe |
AL |
Potamilus amphichaenus (Frierson, 1898) |
Texas heelsplitter |
LA |
Ptychobranchus jonesi (van der Schalie, 1934) |
southern kidneyshell |
AL, FL |
P. occidentalis (Conrad, 1836) |
Ouachita kidneyshell |
AR |
Quadrula cylindrica cylindrica (Say, 1817) |
rabbitsfoot |
AL, AR, KY, TN |
Q. c. strigillata (Wright, 1898) |
rough rabbitsfoot |
KY, TN, VA |
Quincuncina burkei Walker, 1922 |
tapered pigtoe |
AL, FL |
Simpsonaias ambigua (Say, 1825) |
salamander mussel |
AR, KY, TN |
Toxolasma lividus (Rafinesque, 1831) |
purple lilliput |
KY, TN |
T. pullus (Conrad, 1838) |
Savannah lilliput |
GA, NC, SC |
Villosa choctawensis Athearn, 1964 |
Choctaw bean |
AL, FL |
V. fabalis (Lea, 1831) |
rayed bean |
AL, TN, VA |
V. ortmanni (Walker, 1925) |
Kentucky creekshell |
KY |
V. perpurpurea (Lea, 1861) |
purple bean |
TN, VA |
1 Information from U.S. Federal Register 59(219):59008-59010; November 15, 1994. |
||
A recent status review of the mussel fauna in the entire United States revealed significant nationwide declines (Williams et al., 1993). Many mussel species are more depleted than federal lists indicate. In the southeastern states, between 34 percent and 71 percent of the species or populations of species are imperiled, defined here to include endangered, threatened, or of special concern species (Table 4). In spite of the large differences in diversity of mussels among states, the decline of species is ubiquitous in coastal and in Interior Basin rivers. States in the Tennessee River Basin such as Alabama, Tennessee, and Virginia have the highest percentages of imperiled species, whereas coastal states with lower species richness have lower percentages of imperiled species. Best available data indicate that mussel species in the Tennessee River Basin, which includes portions of seven states in the Southeast, are in the most severe decline. Many of the extinct species occurred in the Tennessee and Cumberland rivers and in their major tributaries in Alabama, Tennessee, and Kentucky (Table 5). All 36 species that are presumed extinct occurred in the Southeast, and nearly 40 percent (14) of these were Pleurobema spp. endemic to the Mobile River Basin. As a group, the riffleshells (Epioblasma spp.) have suffered the highest level of extinctions, presumably because of their occurrence in the shoals of mid-size and large rivers that were destroyed by dams and dredging and their intolerance of degraded water quality (Ahlstedt, 1991a). This group of species is seemingly sensitive to physical or chemical changes in habitat suitability, and they are the first to disappear from rivers under anthropogenic disturbance.
Table 4. Status of freshwater mussels in the southeastern United States in 1994. |
||||||
State |
Number of Species |
Number Extinct |
Number Endangered |
Number Threatened |
Number Special Concern |
Total Number Imperiled (Percent) |
AL |
175 |
28 |
51 |
20 |
37 |
136 (78) |
TN |
132 |
17 |
41 |
10 |
29 |
93 (70) |
KY |
102 |
12 |
22 |
7 |
19 |
60 (58) |
GA |
98 |
8 |
23 |
14 |
34 |
72 (73) |
MS |
84 |
0 |
9 |
10 |
21 |
40 (48) |
VA |
80 |
2 |
21 |
9 |
25 |
57 (71) |
AR |
70 |
1 |
6 |
9 |
13 |
29 (41) |
LA |
64 |
0 |
2 |
7 |
13 |
22 (34) |
FL |
51 |
1 |
5 |
10 |
13 |
29 (57) |
NC |
49 |
1 |
6 |
6 |
16 |
29 (59) |
SC |
33 |
1 |
2 |
5 |
10 |
18 (55) |
 A
recent assessment of the aquatic mollusks in North Carolina typifies the extent
of decline in some populations (Scientific Council on Freshwaterand Terrestrial
Mollusks, 1990). Thirty-three (53 percent) of the freshwater mussels in the
state are threatened with extinction, and another 42 species of mollusks are
too poorly known to define their statuses. The collapse of mussel populations
in North Carolina is severe: 62 of 147 populations are reported to be in poor
or very poor condition, and only 19 populations are in very good condition (Rader,
1994). According to Alderman et al. (1992), only 51 of the 147 mussel populations
are likely to maintain viable populations over the next 30 years in North Carolina.
Causes for these declines include waste discharges, nonpoint-source pollution
(especially sediment), reduced instream flow, and competition from exotic species.
A
recent assessment of the aquatic mollusks in North Carolina typifies the extent
of decline in some populations (Scientific Council on Freshwaterand Terrestrial
Mollusks, 1990). Thirty-three (53 percent) of the freshwater mussels in the
state are threatened with extinction, and another 42 species of mollusks are
too poorly known to define their statuses. The collapse of mussel populations
in North Carolina is severe: 62 of 147 populations are reported to be in poor
or very poor condition, and only 19 populations are in very good condition (Rader,
1994). According to Alderman et al. (1992), only 51 of the 147 mussel populations
are likely to maintain viable populations over the next 30 years in North Carolina.
Causes for these declines include waste discharges, nonpoint-source pollution
(especially sediment), reduced instream flow, and competition from exotic species.
Table 5. Species of freshwater mussels in the United States presumed to be extinct. |
||
Scientific Name |
Common Name 1 |
State(s) of Occurrence |
Alasmidonta maccordi Athearn, 1964 |
Coosa elktoe |
AL |
A. robusta Clarke, 1981 |
Carolina elktoe |
NC, SC |
A. wrightiana (Walker, 1901) |
Ochlocknee arc-mussel |
FL |
Elliptio nigella (Lea, 1852) |
winged spike |
AL, GA |
Epioblasma arcaeformis (Lea, 1831) |
sugar spoon |
AL, KY, TN |
E. biemarginata (Lea, 1857) |
angled riffleshell |
AL, KY, TN |
E. flexuosa (Rafinesque, 1820) |
leafshell |
AL, KY, TN |
E. f. florentina (Lea, 1857) |
yellow blossom |
AL, KY, TN |
E. haysiana (Lea, 1833) |
acornshell |
AL, KY, TN, VA |
E. lenior (Lea, 1843) |
narrow catspaw |
AL, TN |
E. lewisii (Walker, 1910) |
forkshell |
AL, KY, TN |
E. obliquata obliquata (Rafinesque, 1820) |
catspaw |
AL, KY, TN |
E. personata (Say, 1829) |
round combshell |
KY |
E. propinqua (Lea, 1857) |
Tennessee riffleshell |
AL, KY, TN |
E. sampsonii (Lea, 1861) |
wabash riffleshell |
KY |
E. stewardsoni (Lea, 1852) |
Cumberland leafshell |
AL, KY, TN |
E. torulosa gubernaculum (Reeve, 1865) |
green blossom |
TN, VA |
E. t. torulosa (Rafinesque, 1820) |
tubercled blossom |
AL, KY, TN |
E. turgidula (Lea, 1858) |
turgid blossom |
AL, AR, TN |
Lampsilis binominata Simpson, 1900 |
|
AL, GA |
Medionidus macglameriae
|
Tombigbee moccasinshell |
AL |
Pleurobema aldrichianum Goodrich, 1831 |
|
AL, GA |
P. altum (Conrad, 1854) |
highnut |
AL |
P. avellanum Simpson, 1900 |
hazel pigtoe |
AL |
P. chattanoogaense (Lea, 1858) |
painted clubshell |
AL, GA, TN |
P. flavidulum (Lea, 1831) |
yellow pigtoe |
AL |
P. hagleri Frierson, 1906 |
|
AL |
P. hanleyanum (Lea, 1852) |
Georgia pigtoe |
AL, GA, TN |
P. hartmanianum (Lea, 1860) |
|
AL, GA |
P. johannis (Lea, 1859) |
Alabama pigtoe |
AL |
P. murrayense (Lea, 1868) |
Coosa pigtoe |
AL, GA, TN |
P. nucleopsis (Conrad, 1849) |
longnut |
AL, GA |
P. rubellum (Conrad, 1834) |
Warrior pigtoe |
AL |
P. troschelianum (Lea, 1852) |
Alabama clubshell |
AL |
P. verum (Lea, 1860) |
true pigtoe |
AL |
Quadrula tuberosa (Lea, 1840) |
rough rockshell |
TN, VA |
1 Not all species have common names. |
||
Table 6. Fingernail clams occurring in the southeastern United States. |
|
Scientific Name |
Common Name |
Sphaerium fabale (Prime, 1852) |
river fingernail clam |
S. occidentale (Lewis, 1856) |
Herrington fingernail clam |
S. striatinum (Lamarck, 1818) |
striated fingernail clam |
S. simile (Say, 1817) |
grooved fingernail clam |
Musculium lacustre (Müller, 1774) |
lake fingernail clam |
M. partumeium (Say, 1822) |
swamp fingernail clam |
M. securis (Prime, 1852) |
pond fingernail clam |
M. transversum (Say, 1829) |
long fingernail clam |
Eupera cubensis (Prime, 1865) |
mottled fingernail clam |
Pisidium dubium (Say, 1817) |
greater eastern peaclam |
P. adamsi Stimpson, 1851 |
Adam peaclam |
P. casertanum (Poli, 1791) |
ubiquitous peaclam |
P. compressum Prime, 1852 |
ridged-back peaclam |
P. equilaterale Prime, 1852 |
round peaclam |
P. fallax Sterki, 1895 |
river peaclam |
P. nitidum Jenyns, 1832 |
shiny peaclam |
P. variable Prime, 1852 |
triangular peaclam |
P. walkeri Sterki, 1895 |
Walker peaclam |
P. punctatum Sterki, 1895 |
perforated peaclam |
P. punctiferum (Guppy, 1867) |
striate peaclam |
The current status and prognosis for the Southeast region’s mussel fauna is grim. Of the 269 species in the Southeast, 13 percent are presumed extinct, 28 percent are endangered, 14 percent are threatened, 18 percent are of special concern, and only 25 percent are considered stable at this time.
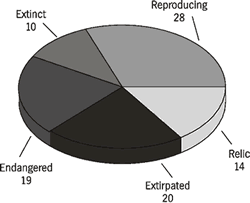
Exploitation of the mussels by humans for food, tools, and ornaments and the deposition of shells in midden piles have provided an excellent archaeological record of species composition during the past 10,000 years. The presence of mussels at archaeological sites has been invaluable in reconstructing prehistoric faunal assemblages and ecological conditions in early times (Bogan, 1990). For example, as judged by the species in shell middens, at least 91 species of mussels occurred in the mainstem Tennessee River during pre-colonial times. That original diversity profoundly changed in this century from human perturbations to the mainstem river. A plethora of dams and degradation of water quality produced irreconcilable changes to the river and its fauna. Results of surveys during the last ten years indicated that only 49 mussel species remain, of which 28 are reproducing and 21 likely are not (Figure 3). Most species with healthy populations were able to tolerate impounded waters and have increased in abundance above pre-impoundment population levels.
The fingernail clams (Sphaeriidae) are small bivalves that live in lotic, lentic, and ephemeral habitats throughout the United States. Of the 38 recognized species (Burch, 1975), about 20 species have ranges that extend into the southeastern states (Table 6). Members of this family are highly adaptable and exhibit an array of species-specific phenotypes to accommodate a variety of abiotic and biotic factors found in aquatic habitats. Some species inhabit stress-prone habitats such as ephemeral ponds and small streams, whereas others seemingly do well in profundal zones of lakes and reservoirs subject to hypoxia.
Fingernail clams, the smallest of freshwater bivalves, release the largest young. A combination of r-selected traits (short life span, early maturity, small size) and k-selected traits (ovoviviparity, low fecundity, large young) seemingly promotes fitness and survival of species that are subjected to periodic stress. Several species prefer coldwater habitats and are restricted to northern climates, whereas species in the Southeast are eurythermal and widespread. Therefore, except for three species of fingernail clams considered to be rare in the Pacific Northwest, none are imperiled in the Southeast. Comprehensive distributional or status reviews of sphaeriids in the United States have not been conducted principally because of the small size, difficulty of identification, and low physical and ecological profile of these clams. More extensive and intensive sampling of permanent and vernal habitats is needed before the occurrence, diversity, and stability of fingernail clam species in the Southeast can be described.
The freshwater gastropod fauna of North America is classified within 14 families and is represented by 516 species (Table 1). Diversity in the southeastern United States consists of 313 species or 61 percent of the native North American freshwater gastropod fauna. Freshwater gastropod diversity was greatest in the Mobile River Basin (118 species), and in the Tennessee River Basin (96 species; Table 7). The Coosa River drainage of the Mobile River Basin was home to four endemic genera (Hydrobiidae: Clappia; Pleuroceridae: Gyrotoma; Planorbidae: Amphigyra, Neoplanorbis). During the past 160 years, the aquatic gastropod fauna of the southeastern United States has been extensively described by Lea (1834-1874) and Goodrich (e.g., Goodrich, 1922, 1924, 1936, 1944a, 1944b), and summarized by Tryon (1873) and Burch (1989). The Hydrobiidae and the Pleuroceridae reached their greatest species richness in rivers of the Southeast. In spite of this great diversity, the ecology and life history of these animals are poorly understood. The freshwater periwinkles (Pleuroceridae) are relatively large snails that live on rocks, cobbles, and bedrock in riffles and shoals and are readily identified by shell characters. Conversely, the hydrobes (Hydrobiidae) are small (<8mm, = less than 0.3 inches) snails that reside in an array of freshwater habitats, from small seeps to large rivers, and typically require anatomical dissection for species identification.
Attention to the status of aquatic mollusk populations was uncommon. Ortmann (1909, 1918) recognized the effects of pollution, acid mine drainage, and dams on the native aquatic fauna. However, that attention was not focused on the decline and disappearance of the aquatic gastropod fauna of the Southeast until the publications of Athearn (1970), Stansbery (1971), Stein (1976) and more recently Bogan and Parmalee (1983), Palmer (1986), and Ahlstedt (1991b). Even now, the life history and ecology of most snail species is poorly understood, and the status of southeastern drainage faunas is virtually unknown. Based primarily on the papers cited above, Turgeon et al. (1988) assembled a list of 23 aquatic gastropods presumed to be extinct in the United States, all of which were endemic to the Mobile River Basin. With 118 species, this basin contained the most diverse aquatic gastropod fauna in the Southeast, and perhaps in the world (Table 7).
Table 7. Summary of the freshwater gastropod fauna in four major river systems in the southeastern United States. |
||||
Taxon |
Cumberland River Basin |
Tennessee River Basin |
Mobile River Basin |
Apalachicola River Basin |
Family: |
||||
|
0 |
0 |
1 |
1 |
|
1 |
1 |
1 |
1 |
|
0 |
0 |
0 |
1 |
|
1 |
4 |
4 |
3 |
|
2 |
20 |
18 |
5 |
|
2 |
3 |
1 |
1 |
|
14 |
50 |
76 |
11 |
|
2 |
2 |
2 |
2 |
|
5 |
5 |
2 |
2 |
|
5 |
6 |
9 |
5 |
|
3 |
7 |
4 |
4 |
Total Species |
35 |
96 |
118 |
36 |
Endangered Species |
0 |
0 |
1 |
0 |
Candidate Species |
8 |
35 |
70 |
3 |
Extinct Species |
0? |
0? |
26 |
0? |
Freshwater gastropods also have been neglected, relative to freshwater bivalves, as candidates for federal protection; only three freshwater gastropods are listed as endangered in the Southeast: Tulotoma magnifica, Athearnia anthonyi, and Pyrgulopsis ogmorphaphe. The tulotoma snail, which is endemic to the Mobile Basin, was presumed extinct until Hershler et al. (1990) discovered several extant populations. As of November 1994, roughly 210 species of 11 families of freshwater gastropods are on the federal list of candidate species. Of these candidate species, 144 (69 percent) occur in the Southeast (Table 8). Taxa from the Southeast account for most of the freshwater prosobranchs and about half of the freshwater pulmonate taxa on the candidate list. Two families (Hydrobiidae, Pleuroceridae) have the greatest number of candidate gastropod taxa.
Table 8. Species of freshwater gastropods on the federal candidate list in the southeastern United States, in 1994. 1 |
||
Taxon |
Common Name 2 |
State(s) of Occurrence |
Prosobranchia (135 Species): |
||
Viviparidae (2 species): |
||
|
cylindrical lioplax |
AL, GA, LA |
|
slender campeloma |
AL |
Hydrobiidae (50 species): |
||
|
|
AL |
|
Blue Spring hydrobe |
FL |
|
Wekiwa hydrobe |
FL |
|
dense hydrobe |
FL |
|
Fenney Spring hydrobe |
FL |
|
Crystal siltsnail |
FL |
|
Ichetucknee siltsnail |
FL |
|
Enterprise siltsnail |
FL |
|
pygmy siltsnail |
FL |
|
ponderous siltsnail |
FL |
|
Seminole siltsnail |
FL |
|
Wekiwa siltsnail |
FL |
|
Cahaba pebblesnail |
AL |
|
umbilicate pebblesnail |
AL |
|
flat pebblesnail |
AL |
|
Briley Creek pyrg |
AL |
|
Spring Creek pyrg |
AL |
|
Flint River pyrg |
AL |
|
Ocmulgee marstonia |
GA |
|
beaver pond marstonia |
GA |
|
olive marstonia |
AL |
|
royal marstonia |
TN |
|
Ozark pyrg |
AR |
|
armored marstonia |
AL |
|
Ouachita pebblesnail |
AR |
|
golden pebblesnail |
AL |
|
anglar pebblesnail |
AL |
|
knotty pebblesnail |
AL |
|
Coosa pebblesnail |
AL |
|
thick-lipped pebblesnail |
AR |
|
stocky pebblesnail |
AL |
|
Tennessee pebblesnail |
AL |
|
hidden pebblesnail |
AL |
|
ovate pebblesnail |
AL |
|
fluted pebblesnail |
AL |
|
granite pebblesnail |
AL |
|
atlas pebblesnail |
AL |
|
dwarf pebblesnail |
AL |
|
moon pebblesnail |
AL |
|
sparrow pebblesnail |
TN |
|
Tallapoosa pebblesnail |
AL |
|
pygmy pebblesnail |
AL |
|
quadrate pebblesnail |
AL |
|
mud pebblesnail |
AL |
|
rolling pebblesnail |
AL |
|
Savannah pebblesnail |
GA |
|
opaque pebblesnail |
AL, TN |
|
panhandle pebblesnail |
NC, VA |
|
channeled pebblesnail |
AR |
|
sculpin snail |
AL |
Pleuroceridae (83 species): |
||
|
Anthony's riversnail |
AL, GA, TN |
|
acute elimia |
AL, TN |
|
mud elimia |
FL |
|
black-crest elimia |
FL |
|
ample elimia |
AL |
|
Lily Shoals elimia |
AL |
|
coal elimia |
TN |
|
walnut elimia |
AL |
|
flaxen elimia |
AL |
|
short-spire elimia |
AL |
|
Cahaba elimia |
AL |
|
spindle elimia |
AL |
|
lacy elimia |
AL |
|
banded elimia |
AL |
|
fusiform elimia |
AL |
|
coldwater elimia |
AL |
|
high-spired elimia |
AL |
|
silt elimia |
AL |
|
gladiator elimia |
AL |
|
constricted elimia |
AL |
|
knotty elimia |
NC, TN |
|
slowwater elimia |
AL |
|
hearty elimia |
AL |
|
ribbed elimia |
AL |
|
round-ribbed elimia |
AL |
|
caper elimia |
AL |
|
engraved elimia |
AL |
|
rough-lined elimia |
AL |
|
nymph elimia |
AL |
|
pupa elimia |
AL |
|
spring elimia |
AL |
|
pygmy elimia |
AL |
|
compact elimia |
AL |
|
brook elimia |
TN |
|
elegant elimia |
TN |
|
mossy elimia |
TN |
|
cobble elimia |
AL |
|
puzzle elimia |
AL |
|
squat elimia |
AL |
|
excised slitshell |
AL |
|
striate slitshell |
AL |
|
pagoda slitshell |
AL |
|
ribbed slitshell |
AL |
|
pyramid slitshell |
AL |
|
round slitshell |
AL |
|
spiny riversnail |
AL, TN, VA |
|
round rocksnail |
AL |
|
agate rocksnail |
AL |
|
oblong rocksnail |
AL |
|
boulder snail |
AL, GA, TN |
|
interrupted rocksnail |
AL |
|
maiden rocksnail |
AL |
|
rotund rocksnail |
AL |
|
lyrate rocksnail |
AL |
|
black mudalia |
AL |
|
knob mudalia |
AL |
|
bigmouth rocksnail |
AL |
|
spotted rocksnail |
AL |
|
plicate rocksnail |
AL |
|
onyx rocksnail |
AL, TN, VA |
|
Coosa rocksnail |
AL |
|
painted rocksnail |
AL |
|
smooth rocksnail |
AL, TN, NC |
|
stripped rocksnail |
AL |
|
armored rocksnail |
AL, IN, KY, TN |
|
knobby rocksnail |
AL |
|
helmet rocksnail |
TN |
|
ornate rocksnail |
AL, KY, TN |
|
rugose rocksnail |
TN |
|
warty rocksnail |
AL, TN |
|
muddy rocksnail |
AL, TN |
|
varicose rocksnail |
AL, KY, TN |
|
rugged hornsnail |
AL, AR, KY, TN |
|
ringed hornsnail |
AL |
|
spiral hornsnail |
AL |
|
corpulent hornsnail |
AL, TN |
|
shortspire hornsnail |
AL, TN |
|
rough hornsnail |
AL, GA |
|
broken hornsnail |
AL |
|
skirted hornsnail |
AL, GA |
|
upland hornsnail |
AL, GA |
|
|
GA |
|
telescope hornsnail |
AL, TN |
Pulmonata (9 Species): |
||
|
||
Ancylidae (3 species): |
||
|
hood ancylid |
AL, FL |
|
domed ancylid |
AL |
|
wicker ancylid |
AL |
Planorbidae (6 species): |
||
|
shoal sprite |
AL |
|
|
AL |
|
|
AL |
|
|
AL |
|
|
AL |
|
magnificent rams-horn |
NC |
Total Aquatic Gastropods: 144 species |
||
1 Information from U.S.Federal Register 59(219):59000-59008; November 15, 1994. |
||
2 Not all species have common names. |
||
Of the aquatic gastropod fauna of four major river basins in the Southeast, the Mobile River Basin had the greatest original diversity but also suffered the greatest destruction and impairment of this fauna. This basin has one endangered species, 70 candidate taxa, and 26 presumed extinct taxa (Table 9). The Mobile fauna has suffered from the effects of damming of the major rivers, sedimentation from poor forestry and farming practices, pollution from industry, and the degradation of water quality as water passes through numerous water treatment facilities. The loss of species richness of the various drainages of the Mobile River Basin ranges from 33 to 84 percent (Table 9). In 1990, the U. S. Fish and Wildlife Service encouraged and supported studies to determine the range distribution and status of this remarkable fauna. Based on results of recent aquatic gastropod surveys by Bogan and Pierson (1993a, 1993b) and U.S. Fish and Wildlife Service personnel, we present a list of freshwater gastropods presumed to be extinct (Table 10). Losses in the Coosa River Basin are most severe. Four genera, with 13 species endemic to the Coosa River drainage, are presumed extinct (Clappia, Gyrotoma, Amphigyra, and Neoplanorbis). Most taxa resided in the main channel of the Coosa River. For example, the extinction of Gyrotoma probably occurred in the mid-1960s with the filling of Logan Martin Reservoir. The last living specimens were collected as the backwaters of the reservoir flooded the rocky shoals occupied by these species (H. Athearn, private museum, Cleveland, Tennessee, pers. comm.). When the series of dams on the Coosa River raised the water over the free-flowing shoals and covered them with silt, most of the fauna probably became extinct.
Table 9. Summary of the aquatic gastropod fauna in the Mobile River Basin. 1 |
|||||||
Taxon |
Alabama River |
Tombigbee River Drainage |
Black Warrior River Drainage |
Cahaba River Drainage |
Coosa River Drainage |
Talapoosa River Drainage |
Mobile River Basin Total |
Gastropod Families: |
|||||||
|
1 |
0 |
0 |
0 |
0 |
0 |
1 |
|
U |
U |
U |
U |
U |
U |
1 |
|
5 |
2 |
0 |
2 |
3 |
1 |
4 |
|
1 |
U |
1 |
3 |
12 |
2 |
18 |
|
U |
U |
U |
U |
U |
U |
1 |
|
7 |
2 |
11 |
22 |
55 |
1 |
76 |
|
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|
U |
0 |
0 |
0 |
6 |
0 |
9 |
|
1 |
U |
1 |
1 |
2 |
0 |
4 |
Approximate total of historic gastropod species diversity |
19 |
8 |
17 |
36 |
82 |
8 |
118 |
Approximate number of collections |
150 |
50 |
100 |
160 |
324 |
16 |
800 |
Number of species found in recent surveys |
3 |
3 |
7 |
24 |
30 |
4 |
80 |
Federally listed endangered species |
1 |
0 |
0 |
0 |
1 |
0 |
1 |
Federal candidate species |
4 |
1 |
6 |
16 |
43 |
2 |
70 |
Number species presumed extinct |
U |
0 |
2 |
4 |
26 |
U |
38 |
Percent decline in fauna |
84 |
62 |
58 |
33 |
63 |
50 |
32 |
1 Data from Bogan et al. (1995). U = unknown or uncertain information. |
|||||||
Table 10. List of the freshwater gastropod species presumed extinct in the Mobile River Basin.1 |
|
Taxon |
Common Name2 |
Hydrobiidae: |
|
|
Cahaba pebblesnail |
|
umbilicate pebblesnail |
Pleuroceridae: |
|
|
short-spire elimia |
|
closed elimia |
|
fusiform elimia |
|
|
|
high-spired elimia |
|
constricted elimia |
|
hearty elimia |
|
|
|
ribbed elimia |
|
|
|
rough-lined elimia |
|
pupa elimia |
|
pygmy elmia |
|
cobble elimia |
|
excised slitshell |
|
striate slitshell |
|
pagoda slitshell |
|
ribbed slitshell |
|
pyramid slitshell |
|
round slitshell |
|
agate rocksnail |
|
oblong rocksnail |
|
interrupted rocksnail |
|
maiden rocksnail |
|
rotund rocksnail |
|
lirate rocksnail |
|
black mudalia |
|
bigmouth rocksnail |
|
Coosa rocksnail |
|
|
|
striped rocksnail |
Planorbidae: |
|
|
shoal sprite |
|
|
|
|
|
|
|
|
1 Data from Bogan et al. (1995). |
|
2 Not all species have common names. |
|
The aquatic gastropod fauna of the southeastern United States is continuing to decline. Dams on the major rivers of the Southeast eliminated these animals from much of their former range such that only relict populations survive immediately below some of the dams. The fragmented ranges and the isolated populations result in species becoming susceptible to extirpation and extinction.
The extinction, extirpation, or decline of most freshwater mollusks can be attributed to biological attributes and ecological requirements that make species particularly vulnerable to anthropogenic effects. Freshwater mussels have an unusual reproductive cycle; the larval stage (glochidium) is an obligate parasite on the gills or fins of host fishes. Host specificity is the rule rather than the exception in most freshwater mussels (Hoggarth, 1992). Gravid female mussels release tens of thousands to several million glochidia, depending on the species and the size of the female (Surber, 1912; Coker et al., 1921; Yeager and Neves, 1986; Hove and Neves, 1994). Although the number of larvae produced is high, few glochidia contact and attach to the appropriate host fishes during this r-selected stage in the life cycle. Thus, the timely presence and abundance of appropriate fishes to complete the reproductive cycle is critical to the continued existence of freshwater mussel species. Because less than 20 percent of mussel species in the Southeast have known host fishes, this potential reproductive bottleneck cannot be evaluated until hosts are identified for the imperiled mussels. Any factor that alters the natural assemblage of fishes can threaten the viability or composition of the associated mussel assemblage.
Habitat loss and degradation affects mollusks directly by reducing population sizes and inhibiting long-term reproductive success. The degrees of rarity of nearly all species stem from anthropogenic losses and alterations of habitats. Because these perturbations to the biology and ecology of mollusks are documented, we provide a summary of those factors most lethal to the continued existence of mollusk populations. The dynamic changes in mollusk assemblages in rivers and reservoirs is evidenced by declining diversity, changes in species composition, and lowered abundance of some species resulting from acute and chronic alteration and degradation of habitat suitability for native species. It is these insidious factors that perpetuate the downward spiral of distribution and diversity of our native mollusks.
The effects of dams and resultant impoundments are detrimental to riverine fishes and freshwater mollusks. Changes in mussel faunas are perhaps best documented in the Tennessee River, impounded by a series of 36 multi-purpose dams on the mainstem and on major tributaries. Reductions in the diversity and abundance of mussels are principally attributed to habitat shifts caused by impoundment. Upstream of dams, the change from lotic to lentic waters, increased depths and sedimentation, decreased dissolved oxygen, and the drastic alteration in resident fish populations inevitably can jeopardize the survival of some mussels and their reproductive success. The loss of benthic host fishes and the spatial separation of remaining pelagic and littoral fishes from residual mussel populations preclude the sympatric requirement for glochidial infestations. Downstream of dams, fluctuations in flow regime, scouring, seasonal dissolved oxygen sags, reduced water temperatures, and changes in fish assemblages also can jeopardize the survival and reproductive success of many mollusk species. This tailwater effect may extend for many kilometers downstream and result in the gradual attrition of environmentally sensitive mollusks. Because mussels are thought to be the longest-lived freshwater invertebrates, with a longevity of more than 100 years for some species, population declines due to poor reproductive success may continue for decades. Thus, the extirpation of species is a prolonged event, lagging decades behind the factors directly responsible for attrition of the fauna.
Effects of impoundments on the mussel fauna of coastal rivers are similar to those reported for the Tennessee River. In the Tombigbee River, a large Coastal Plain river in western Alabama and northeastern Mississippi, Williams et al. (1992) reported a loss of about 70 percent of the preimpoundment fauna. A preimpoundment mussel survey of the Black Warrior River is incomplete, but it appears that the loss in species richness is similar to that reported in the Tombigbee River. Most of the species of mussels that survive in Coastal Plain impoundments are the same as those that survive impoundments in upland areas such as the Tennessee River.
Mussel surveys before and after reservoir construction on several rivers in the Southeast attest to the drastic changes in mussel fauna caused by habitat shifts. In the Tennessee River, the Pickwick Dam inundated perhaps the most diverse assemblage of mussels in the world, about 70 species in 31 genera. The disappearance of nearly half of the species seems directly attributed to destruction of riverine habitat. Other species suffered a similar but prolonged fate for lack of reproductive success. At Muscle Shoals, Ortmann (1925) reported 69 mussel species before the Wilson Dam was constructed. Mussel diversity in this river reach declined to 44 species in 1968 (Isom, 1969), and to fewer than 30 species now. From the mouth of the Tennessee River upstream to Fort Loudoun Dam (963 km, = 598 miles), only tailwaters and overbanks in the lower river remain as suitable habitat for riverine species. There is little or no reproduction of mussels in the Tennessee River upstream of Fort Loudoun Dam, perhaps because the upper river lacks the necessary flow conditions for reproduction by riverine species. The loss of riverine species was accompanied by the invasion of mud-tolerant species into the reservoirs through either stocking of fishes with incidental infestations, natural fish movements upstream, or the seeding of reservoirs with commercially important species by entrepreneurial mussel harvesters. Irrespective of the path of entry, the reservoir-tolerant species (e.g., Anodonta spp., Potamilus spp., etc.) greatly increased in abundance and now dominate the mussel fauna in many reservoirs (Ahlstedt and McDonough, 1993). The loss of reservoir-intolerant species meant an end to the indigenous and endemic fauna, and set the stage for invasive non-native species to proliferate in artificial but suitable environments.
The historic species composition of the Tennessee River indicates that at least 49 species of mussels or their host fishes are intolerant of reservoir conditions or the subsequent physico-chemical changes in the river (Figure 3). Conversely, about 28 species of native and non-native mussels increased in abundance to occupy primarily the overbank areas where conditions were best suited to successful reproduction and juvenile survival in the soft sediments. As judged by the longevity of some species, the mussel assemblage continues to approach a climax community, with some semblance of long-term stability.
Impoundments have had similar detrimental effects on freshwater gastropods, although poorly documented. In the Mobile River Basin, 38 species of snails are presumed extinct, primarily as a result of impoundment (Table 10).
Although freshwater mussels have been commercially harvested since the late 1800s, there is no evidence of permanent damage to populations or species due to this industry. From the beginning of harvesting to provide shells for a burgeoning pearl button industry and now for the cultured pearl trade, the dozen or so commercial species have persevered the waxing and waning of harvest effort by musselers. The most sought after commercial species such as the ebonyshell (Fusconaia ebena), the threeridge (Amblema plicata), the washboard (Megalonaias nervosa), and others are widespread and abundant. Harvests in the Mississippi and Tennessee river systems were most intense, and some river reaches were exploited to economic overharvest before being allowed to slowly recover (Claassen, 1994). Similar problems occurred in Gulf Coast rivers, but the fishery was less intense because of low-quality shell and smaller mussel populations. Reduced catch per unit effort and the law of diminishing returns functioned to prevent biological overharvest of healthy mussel beds. These populations and their essential host fishes remained in sufficient abundance to begin the gradual recovery from economic overharvest. As a result of this early onslaught and the realization that mussels were a renewable but exhaustible resource, state fish and wildlife agencies began to actively manage this fishery in the 1960s through restrictions on species, size, gear, location, and time of year. Most states that allow commercial harvest now have regulations to manage the fishery and personnel to monitor and enforce these regulations.
Concurrent with commercial exploitation of mussel populations in the Mississippi Basin during the early 1900s, a profusion of dams and reservoirs constructed by the Tennessee Valley Authority, U.S. Army Corps of Engineers, and public utilities created lentic habitats preferred by several commercial species (Ahlstedt and McDonough, 1993). The natural invasion or human transfer and proliferation of desirable species to these new impoundments supplemented the availability of commercially exploitable populations. This cornucopia of economically valuable species, however, came at the expense of native biodiversity.
Table 11. An assessment of water quality in rivers of the southeastern United States in 1992.1 |
||
State |
River Kilometers Assessed 2 |
Kilometers of Impaired Water (Percent) |
Alabama |
19,667 |
5,507 (28) |
Arkansas |
11,939 |
6,089 (51) |
Florida |
12,693 |
4,569 (36) |
Georgia |
6,486 |
4,605 (71) |
Kentucky |
15,579 |
5,141 (33) |
Louisiana |
14,542 |
10,761 (74) |
Mississippi |
57,366 |
53,924 (94) |
North Carolina |
56,096 |
35,340 (63) |
South Carolina |
6,326 |
1,771 (28) |
Tennessee |
17,317 |
9,178 (53) |
Virginia |
28,733 |
5,747 (20) |
1 Data from U.S. Environmental Protection Agency (1994). |
||
2 One kilometer equals 0.62 miles. |
||
The condition of streams and rivers in the United States has been monitored
by the United States Environmental Protection Agency (EPA) for roughly 20 years.
Prior to this national monitoring of river basins, assessments of ecological
health were limited to rivers of high priority in a state or to interjurisdictional
rivers. The most recent biennial assessment by EPA was of the quality of about
18 percent of all United States river miles (U.S. Environmental Protection Agency,
1994). Of the roughly 643,000 miles (1,028,800 km) of assessed rivers, 56 percent
fully supported their designated uses of fishable and swimmable (Figure 4).
The remaining 44 percent of river miles were threatened (6.7 percent), only
partially supported (25.7 percent), or did not support (13.2 percent) designated
uses. In the southeastern states, the degree of impairment of public waters
varied tremendously (Table 11). Although reported values represent a limited
sample of waters per state, the general impression is that many of the monitored
rivers in the Southeast have impaired water quality. Pollutants that contribute
to the impairment of water quality in rivers are principally sedimentation (45
percent) and excess nutrients (37 percent) (Figure 5). Other causes of impairment
originate from point- and nonpoint-sources of discharge. 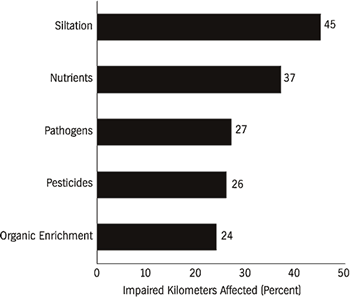
The most widely reported source of pollution to rivers is agriculture (Figure 5). Traditional farming practices, feed-lot operations, and associated poor land-use practices contribute many pollutants. Agriculture affects 72 percent of impaired river kilometers in the United States, yet it has been largely neglected in legislative efforts to curb pollution to public waterways. Although the U.S. Congress explicitly referenced land-use requirements in the Water Pollution Control Act of 1972 to control nonpoint-source pollution (Selzer, 1994), the legislation has been largely ineffective on agriculture. Some areas in the Southeast, such as southwestern Georgia, have experienced severe losses of topsoil and nutrient additions to local streams due to agriculture. Most major forests in this region were cut by the late 1920s, and intensive agriculture ensued. The Flint River watershed in the center of this agricultural belt has been greatly affected by this landscape transition. Soil erosion and runoff of fertilizer and pesticides into groundwater and surface water have had a profound effect on water quality in the river and on the indigenous biota (Patrick, 1992). Nationwide decreases in lead and fecal coliform bacteria in streams have been countered by increases in nitrate, chloride, arsenic, and cadmium concentrations (Smith et al., 1987). These water quality changes are seemingly the result of improved sewage treatment and unleaded gas consumption, and increased use of fertilizers and highway salt. The increased use of fertilizer and subsequent eutrophication is thought to have been a dominant influence in water quality changes during the 1980s. Fuller (1974) provided an excellent but somewhat outdated review of the effects of pollutants on freshwater bivalves.
National statistics on water quality problems adequately reflect the situation in the Southeast, as judged by technical documents such as 305b reports submitted to the EPA in alternate years by states. Although too voluminous to present in this assessment of freshwater mollusks, trends in water quality in Tennessee, a centrally located and significant state for mollusk diversity, provide a suitable southeastern perspective (Tennessee Department of Environment and Conservation, 1990). Most stream kilometers and lake hectares are clean enough to fully support designated uses. Degradation to streams from agricultural crops is most intense in western Tennessee, whereas runoff from animal holding lots is troublesome throughout the state. Mining effects are most severe in the Cumberland Plateau region of eastern Tennessee. As a result of point-source control of pollutants after enactment of the Tennessee Water Control Act of 1972, water quality improved drastically. Statewide, 83 percent of streams have stable water quality, four percent have improved quality, and 13 percent are continuing to degrade. The cause for most degradation of water quality is attributed to nonpoint effects. Hence, national legislation such as the Clean Water Act of 1977 has drastically and significantly improved the regulation of point-source discharges. One of the goals of this act was the maintenance and restoration of the chemical, physical, and biological integrity of the nation’s waters. Progress toward this goal is well under way. Still lacking are the legislative means to significantly reduce nonpoint runoff from agricultural and urban areas. Support within each of the states for mandatory Best Management Practices (BMPs) would go a long way toward curbing the dominant nonpoint problems that continue to degrade water quality and jeopardize all aquatic biodiversity in southeastern streams.
Voluminous research and management experience have clearly documented the interdependence
of terrestrial and aquatic ecosystems for the overall health of biota (Pajak
et al., 1994). However the implementation of this knowledge through effective
and comprehensive policy change has been egregiously slow. A quantum leap in
progress occurred with passage of the Farm Bill (Food Security Act of 1985)
and its subsequent reauthorization in 1990. Through its Conservation Reserve
Program (CRP), more than 14.6 million hectares (= 36 million acres) of marginal
farmland have been retired from production (Agricultural Stabilization and Conservation
Service, 1993). The estimated reduction in soil erosion and nonpoint-source
pollution exceeds 694 million tons of soil per year, with fish and wildlife
habitat improved in a few southeastern states. Managing for clean water, fish
and wildlife habitats, and other nontraditional products may be the most beneficial
use for inherently marginal agricultural land, riparian zones, and other ecologically
sensitive areas in watersheds. The maintenance of buffer strips along streams
and rivers is particularly crucial to the welfare of freshwater mollusks. Forested
buffer strips (30-50 m wide, = 100-164 feet wide) and grass buffer strips (4.6-27
m wide, = 15-89 feet wide) can reduce nitrate and phosphorus concentrations
in surface runoff by 79-98 percent and 54-84 percent, respectively (Osborne
and Kovacic, 1993). Similarly, 95 percent of soil lost through cropland runoff
can be retained with a 9 m wide (30 feet) vegetation strip (Schultz and Cruse,
1992). However, because less than one percent of CRP land occurs in close proximity
to water resources, the benefits of this program to aquatic ecosystems have
been more trickle down than overflow economics. Until aquatic biologists participate
directly in agricultural programs designed to produce and conserve resources
of societal benefit, the degradation of public waters through runoff from private
property will continue to jeopardize the existence of silt-intolerant species
and their communities.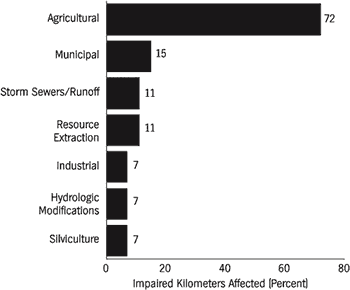
Declines in mollusk populations from water pollution were chronicled in the 19th and early 20th centuries (Lewis, 1868; Ortmann, 1909; Baker, 1928), when the problems from industrial effluent became widespread. Mollusks can avoid or tolerate short-term exposures to toxic chemicals by valve or operculum closure, but most cannot tolerate chronic exposures to contaminated water. Havlik and Marking (1987) summarized the few available data on body burdens and toxicity levels of various contaminants. Because many adult mollusks can avoid exposure, they are not suitable bioassay organisms for standard toxicity tests. However, pulmonate snails and the early life stages of freshwater mussels are more readily suited to toxicity testing, especially because early life stages tend to be more sensitive than adults. The recent use of glochidia and juvenile mussels in bioassays may foster standardized techniques and acceptance of these and other mollusks (e.g., Corbicula fluminea) for toxicity testing (Johnson, 1990; Keller and Zam, 1991; Goudreau et al., 1993; Jacobson et al., 1993). Determination of sensitivities of mussels to particular contaminants, relative to the standard aquatic bioassay organisms such as the zooplankter Ceriodaphnia dubia and fathead minnow Pimephales promelas, also would help establish general and site-specific criteria for water quality in rivers where no suitable surrogates are available. The identification of appropriate surrogate species is important for routine use in standard bioassays to establish environmentally safe criteria for contaminants or whole effluents discharged into waterways containing biologically significant mollusk populations. As judged by the decline and degree of rarity of mollusks in southeastern rivers, criteria to protect this faunal group are urgently needed.
Sediment and contaminants in nonpoint-source runoff are insidious factors in aquatic ecosystem degradation. Sediment degrades water quality and substratum suitability for mollusks by clogging gills, reducing feeding efficiency, and eventually covering algae scraped from rocks by snails or smothering mussels after sufficient accumulation (Ellis, 1936; Marking and Bills, 1979; Kat, 1982; Willis et al., 1994). Field and laboratory studies implicated silt and sedimentation from agriculture, mining, and other land-use practices in the decline of mollusks in streams (Ellis, 1931; Coon et al., 1977; Wilber, 1983; Aldridge et al., 1987). The subtleties of these effects usually are not documented because erosional silt enters waterways during storm events and from construction sites lacking suitable Best Management Practices. However, these periodic additions of sediment have profound effects on long-term sustainability of mollusk populations. For example, depleted snail populations in pools of the upper Powell River, Virginia, were found on the surface of cobbles and boulders in spring but on the underside of rocks in summer. Presumably, the accumulation of silt from abandoned mined lands on cobbles and boulders in summer inhibits the growth of algae and discourages the attachment and grazing by snails. Thus, seasonal pulses of erosional silt are precluding the establishment of healthy populations of resident gastropods in riffle and run habitats.
The southern Appalachians are characterized by high topographic relief with steep slopes and high gradient streams. Coal mining in this region includes contour strip mining, mountain-top removal, and limited deep and longwall mining operations. Surface mining has degraded many streams that drain the Appalachian coal fields in southwestern Virginia, eastern Tennessee, eastern Kentucky, and northern Alabama (Barnhisel and Massey, 1969; Curry and Fowler, 1978). Because coal deposits are numerous in the Cumberland Plateau region (an area noted for its diversity and endemism of aquatic fauna), many species of mollusks have been extirpated from headwater streams where mining has been most intense. The market price of coal does not portray the array of external costs to society and to ecosystems associated with mining (Herlihy et al., 1990; Cullen, 1993). Surface mining strips away the overburden and exposes underlying coal seams; the result is typically dysfunction of vegetation, increased sedimentation, and mine drainage. The formation of sulfuric acid from exposure of iron pyrite acts as a solvent for metallic minerals bound in rock strata. Subsequent runoff may contain high levels of heavy metals, determined by local geology, that have toxic effects on aquatic fauna (Stiefel and Busch, 1983; Caruccio et al., 1988). Ahmad (1973) estimated that nearly 29,000 km (18,020 miles) of streams in Appalachia have been destroyed for decades to come from mining activities.
Erosion from mined slopes and haul roads has increased sedimentation and turbidity in streams. Branson and Batch (1972) recorded a 90 percent reduction in benthos in two Kentucky streams that receive low-level mine drainage and a high level of siltation and turbidity from spoil banks. From comparisons of flora and fauna in streams of mined and undisturbed watersheds, overwhelming evidence has been presented of the large declines in biological diversity and abundance of organisms (Vaughan, 1979; Matter and Ney, 1981).
Historic and recent accounts of surface mining effects on aquatic mollusks are replete in the scientific literature. Neel and Allen (1964) attributed the decline of riffleshells (Epioblasma spp.) in the Cumberland River system to increased coal mining, particularly in the Big South Fork watershed. Starnes and Starnes (1980) indicated coal mining as the cause for the disappearance of the little-wing pearlymussel (Pegias fabula) and for the rapid decline of other mollusks in the Big South Fork. Stansbery (1969) attributed declines in diversity and density of mussels below the Cumberland Falls to acid mine drainage from upstream mines. Branson et al. (1984) reported the extirpation of mollusks from two streams in Kentucky affected by surface mining. Williams (1969) observed deposited coal waste on the periostracum of mussels in Kentucky but could not determine whether such debris was detrimental to mussels. In Virginia, aquatic mollusks were eliminated in the North Anna River below an acid mine drainage outfall (Simmons, 1972). In the upper Powell River, Virginia, freshwater mussels and gastropods were eliminated for about 24 river km (about 15 miles). Ortmann (1918) collected mussels as far upstream as Powell River Mile (PRM) 178.2, but subsequent surveys recorded sites above PRM 140 to be seriously degraded by coal waste and sediment disposition, and no mussels were found upstream of PRM 165 (Ahlstedt and Brown, 1979; Neves et al., 1980; Dennis, 1981; Wolcott and Neves, 1994).
Although the Surface Mine Control and Reclamation Act of 1977 (SMCRA) has done much to reduce severe degradation caused by erosion and acid mine drainage (Doll, 1988), increased demand for coal in the 1980s expanded mining in existing coal fields and in previously undisturbed areas. Documentation of declines in freshwater mollusks in the Southeast continue to appear in the literature and are attributed principally to sedimentation from active mines. Houp (1993) reported excessive sand deposits from coal mining in the North Fork of the Red River, Kentucky, and described how chronic sedimentation can gradually alter species composition of mussels through habitat degradation, shell erosion, and reproductive failure. Anderson (1989) evaluated the mussel assemblages in four streams of the Cumberland River drainage where coal mining began in the 1980s. In situ toxicity tests indicated lethal conditions in stream segments below mined areas regardless of pH conditions. He concluded that surface mining regulations are inadequate to protect mussels in this drainage. Thus, although environmental safeguards are much improved, negative effects of coal mining continue to jeopardize mollusks in headwater streams.
One effect of coal mining that has not been addressed in previous reports is the treatment and disposal of hydraulic oils by longwall mining operations. Hydraulic oil emulsions in the hydraulic jacks and other underground equipment are acutely toxic to aquatic life. These oils contain a variety of additives, including biocides, to prolong their use and purity in hydraulic systems. Depending on the oil product, LC50 values on standard bioassay organisms demonstrated toxicity levels ranging from parts per million (mg/L) to parts per billion (mg/L) (Biological Monitoring, Inc., 1991, 1993). Results of these studies also indicate that available treatment technologies can profoundly reduce the concentration of emulsion present and resulting effluent toxicity. Contaminated minewater treatment and effluent monitoring by fluorometry should be mandatory requirements imposed by federal and state regulatory agencies responsible for discharges from longwall mining operations.
Other types of mining activities also affect aquatic mollusks. Ortmann (1924) suggested that phosphate and iron mines in the Duck River watershed, Tennessee, caused a precipitous decline in mussel populations. Reduced growth rates have been reported in mussel populations in the Tennessee River downstream of sand and gravel mining operations (Yokley, 1976). Hartfield (1993) has reported mussel extirpations associated with offsite impacts of instream and floodplain sand and gravel mines. In western North Carolina, the mining of industrial minerals (kaolin, mica, feldspar) caused significant water quality problems in receiving streams (Tennessee Valley Authority, 1971; U.S. Environmental Protection Agency, 1977; Duda and Penrose, 1980). The North Toe River has been categorized as a biological desert (Tennessee Valley Authority, 1971) with such severe sedimentation that the Davy Crockett Reservoir, more than 200 river km (124 miles) downstream of the mines, is now filled with sediment and is inoperable for power generation.
Dredging and channelization contribute to stream channel instability as running water seeks its base level of gravitational flow. The destructive effects of channelization and extensive dredging include accelerated erosion, substratum instability, and the loss of habitat heterogeneity for fishes and benthic invertebrates. Maintenance dredging for navigation and gravel dredging in large rivers has been a perpetual problem for sedentary mollusks that are displaced and killed in dredge spoils. According to reports of commercial mussel harvesters, dredging causes some of the most serious effects on mussel beds in the Mississippi, Ohio, Tennessee, and other rivers that sustain commercial harvest. Except for consideration given to federally listed endangered species, dredging projects have little regard for resident aquatic fauna, destroying faunal habitat and promoting community instability.
Although the volume of literature demonstrating negative on-site and off-site environmental and economic consequences of dredging for navigation and flood control is substantial (Smith and Patrick, 1991), these activities continue in the Southeast. For example, a channel maintenance project ostensibly for flood control is currently planned for the Big Sunflower River, which drains the western portion of the Yazoo River Basin, located in Mississippi. The Yazoo River Basin historically supported a diverse and dense assemblage of freshwater mussels in every major tributary. The Big Sunflower River is unusual in this portion of the Mississippi River alluvial plain, in that much of the mainstem river has not been subjected to dredging and continues to support a fairly diverse and dense mussel assemblage. Miller et al. (1992) and Miller and Payne (1994) have reported 32 species of mussels from the river, and densities exceeding 100 mussels per m2 (about nine per square foot) at many locations. The channel maintenance project includes dredging much of the mainstem river where mussels are found. This project likely will have little long-term benefit towards flood control, but will undoubtedly destabilize the river bottom, destroy mussel beds, and jeopardize the ecology of this river.
Endangered mussels of big rivers such as the white wartyback (Plethobasus cicatricosus), orange-foot pimpleback (P. cooperianus), pink mucket (Lampsilis abrupta), and ring pink (Obovaria retusa) have been under siege for decades by navigational dredging mostly by the U.S. Army Corps of Engineers. Even the presence of federally endangered species does not prevent the modification of habitats where these animals reside. For example, the navigation channel below Pickwick Dam on the Tennessee River was slated for dredging, even though this river reach is a state-designated mussel sanctuary with federally endangered species present. After consultation with the U.S. Fish and Wildlife Service, the project was approved contingent on 1992-1994 sampling efforts to collect and translocate rare mussels from the affected zone. Tennessee Valley Authority divers nearly completed their transect sampling to remove mussels in 1994 before the dredging began. Although many animals were collected and saved from destruction, there is something inherently troublesome with human biases in the sacrifice of habitats for some species (e.g., invertebrates) but not for others. For purposes of analogy, if this project was to remove vegetation from the right-of-way for an existing powerline, where red-cockaded woodpeckers had taken up residence, would the nesting trees and surrounding woodlands be removed? Aren’t the trees used by woodpeckers analogous to the substratum occupied by aquatic mollusks? The legislative goal of equal treatment under the law continues to elude the non-charismatic creatures of America’s heritage.
A more insidious result of channel degradation is head cutting, the effect of base leveling of the modified channel on upstream reaches. Hartfield (1993) noted two primary sources of headcuts: channelization for flood control or navigation and gravel mining. Changes in stream channel, slope, discharge, and other physical factors are typically accompanied by erosional processes that adjust and maintain channel equilibrium (Smith and Patrick, 1991). The longitudinal profile or change in elevation over distance of a stream is typically concave, and has a decreasing slope from the upstream (eroding) reaches to the lower (depositional) reaches. This profile is modified by local topography and stream-bed features, where an abrupt change in slope (knickpoint) can occur. Because of increases in transport capacity through downstream dredging, degradation through erosion will progress upstream until some knickpoint is reached. Water movements and erosion are typically pronounced at this location and eventually lead to failure of the overlying material and upstream migration of the knickpoint (Gordon et al., 1992). Thus modifications to the downstream channel can have repercussions far upstream, particularly in coastal plain streams with easily erodible substrata.
Hartfield (1993) described the following characteristics of recently headcut streams: extensive bank erosion; wide, degraded channels; extensive bank sloughing; unconsolidated and shifting sediment; perched tributaries at low flow; and the absence of characteristic mature trees of stable riparian zones. Freshwater mussels are particularly susceptible to headcuts because they are immobile and highly subject to channel instability. Hartfield (1993) reported headcut effects on mussels and snails to include federally endangered species in several rivers in Mississippi and Louisiana. Although the full extent of faunal losses is unknown, effects on rare species and their habitats are inevitable. The threatened inflated heelsplitter (Potamilus inflatus) is jeopardized by gravel mining in the Amite River, Mississippi. The black clubshell (Pleurobema curtum), heavy pigtoe (P. taitianum), and southern combshell (Epioblasma penita) are threatened with extinction; and the southern clubshell (P. decisum), ovate clubshell (P. perovatum), orange-nacre mucket (Lampsilis perovalis), and Alabama moccasinshell (Medionidus acutissimus) are in danger of extirpation from remaining habitats in the Tombigbee River drainage due to habitat degradation. The Big Black rocksnail (Lithasia hubrichti) is on the verge of extinction because of channel degradation in the Big Black River, Mississippi.
The introduction or translocation of non-native freshwater mollusks into the southeastern United States has been adversarial to native assemblages. The Asian clam (Corbicula fluminea) invaded the Gulf Coast and Interior Basin rivers in the 1960s and spread rapidly throughout every major drainage in the southern United States. Its prolific reproductive ability and ecological role as a filter-feeder are traits conducive to competitive interactions with native mussels. Although debatable evidence has been presented to document competitive exclusion between these bivalve taxa (Sickel, 1986), substratum space and food utilization are requisite resources that could be limiting in rivers with high densities of Asian clams. Juvenile freshwater mussels may become victims of stress from the highly mobile and abundant young Asian clams. After tens of millions of years of evolutionary speciation and adaptation to river systems in the Southeast, it is possible that native mollusks have filled all suitable niches in the benthos. Effects of competitive interaction are difficult to document and research is required to identify factors allowing coexistence or causing exclusion among mollusk species.
Several species of exotic snails have infiltrated aquatic habitats in the Southeast (Table 12), but there has not been the proliferation or degree of invasion that occurred with the Asian clam. Most of these exotic snails have specific habitat requirements, such that their distribution and competitive interaction has been limited. At this time, there is little evidence to suggest past or future significant changes to diversity of native gastropods because of these nonindigenous species.
Table 12. Species of introduced freshwater mollusks in the United States. |
|
Taxon |
Common Names |
Bivavia: |
|
|
< |
|
greater European peaclam |
|
Henslow peaclam |
|
humpback peaclam |
|
European fingernailclam |
|
|
|
Asian clam |
|
|
|
zebra mussel |
|
quagga mussel |
Gastropoda: |
|
|
|
|
Chinese mysterysnail |
|
Japanese mysterysnail |
|
|
|
giant rams-horn |
|
|
|
red-rim melania |
|
fawn melania |
|
quilted melania |
|
|
|
big-ear radix |
|
|
|
glass physa |
|
|
|
bloodfluke planorb |
|
rusty rams-horn |
|
ridged rams-horn |
|
crested rams-horn |
Of greatest concern now and in the future is the massive invasion of the exotic zebra mussel (Dreissena polymorpha) and its sister species the quagga mussel (Dreissena bugensis) into North America (Rosenberg and Ludyanskiy, 1994). The zebra mussel presumably was transported to the Great Lakes in 1986 in ballast water derived from a European port. It ravaged native mussel populations in Lake St. Clair and Lake Erie (Schloesser and Kovalak, 1991; Hunter and Bailey, 1992), entered the Illinois River from Lake Michigan, and rapidly spread like a plague throughout the entire Mississippi River Basin (Ludyanskiy et al., 1993). In only nine years since its introduction into U.S. waterways, the zebra mussel now infests rivers, reservoirs, and lakes in 19 states.
The fate of native mussels in the Illinois River will be the test case for what can be anticipated in many southeastern rivers due to zebra mussels, and the status of native unionids and snails in this river is foreboding to other rivers (Blodgett et al., 1994; Tucker, 1994). From 1989-90 canals linking Lake Michigan and the Illinois River became infested, and zebra mussels were first collected at multiple sites in the river’s mainstem in 1991. In 1992, they spread throughout the entire river, achieving densities of nearly 1,600 per m2 (149 per square foot). By 1993, maximum densities approached 61,000 per m2 (5,667 per square foot), and a gradient of increasing numbers was progressing downstream. Zebra mussels were attached to all solid substrates, including native unionid mussels and gastropods. From 4 percent to 100 percent of unionids had zebra mussels attached, up to about 1,500 per native mussel. Mortalities of unionids from attachments were apparent in 1993, caused ostensibly by the occlusion of unionid valves preventing normal respiration and feeding activity. As judged by the well-documented effects in this river, many native mussel species may soon be extirpated from the Illinois River and from other rivers in the Mississippi Basin with suitable environmental conditions for this prolific and deadly pest.
The transport of this zebra mussel into the Tennessee River up to the head of commercial navigation (Knoxville, Tennessee) was complete by 1993. Commercial barge traffic was obviously the major vector of transport to large rivers, but pleasure craft will probably continue to spread the zebra mussel into smaller rivers and reservoirs. It is almost inevitable that zebra mussel-infested waters will occur in nearly all southeastern states, and that some level of effect will occur to native mollusks. The zebra mussel will probably be the final nail in the coffin of several federally protected mussels that succumb to infestations in large rivers. Other commercial and rare mussels may require endangered species status if the zebra mussel infestations extirpate river and reservoir populations and drastically reduce the ranges of one-time widespread big-river species. The urgency of protection and conservation of native mussels cannot be overemphasized. Natural resource agencies in the Southeast must be proactive in efforts to prevent the wholesale extinction of mussels in the direct path of the zebra mussel invasion.
The United States has had only piecemeal legislation to regulate the intentional importation of fish and wildlife species. The Lacey Act was the primary law for excluding harmful imports that posed a threat "to humans, aquaculture, horticulture, forestry, or to wildlife or the wildlife resources of the United States." However black-listed species under this legislation included very few species of finfish or shellfish. The Nonindigenous Aquatic Nuisance Prevention and Control Act of 1990 was the first legislation authorizing the U.S. Fish and Wildlife Service and the National Oceanic and Atmospheric Administration to issue regulations preventing the unintentional introductions of aquatic nuisance species such as the zebra mussel (Office of Technology Assessment, 1993). Regulations under this act will hopefully prevent the incidental importation of other aquatic mollusks harmful to native species. However, there is negligible federal involvement in the interstate transfers of nonindigenous fish and wildlife. Although states assume primary responsibility for interstate trafficking of nonindigenous species, most have insufficient standards of review and enforcement. Taken together, regulations at the federal and state levels are inadequate to exclude or regulate the import or transfer of harmful mollusks among states. It is essential therefore that new national legislation be prepared to increase the rigors of protocol for importation and release of nonindigenous animals and to establish state roles and responsibilities for transfers of such species. To prevent the intentional introduction of undesirable non-native aquatic species, the Lacey Act should be strengthened.
Rivers of global significance in the Southeast have been permanently altered to provide human benefits measured in mere seconds of evolutionary time. The direct exploitation of rivers, which accelerated rapidly in the 1930s for water supply, flood control, hydropower, and navigation, has nearly ended. However, what remains are disjunct river reaches in most states with faunas isolated either by dams, pollution blocks, or other barriers preventing holistic recovery. The Federal Energy Regulatory Commission (FERC) has regulatory authority for relicensing hydropower dams, as well as authority to require the eventual decommissioning of old dams that have outlived their utility. For functional dams, conservation improvements to include fish passage, epilimnetic discharges, and minimum stream flows for fauna and for recreation are highly desirable. Too many dams in the Southeast continue to impede the migrations of fish and recovery of mollusks and other taxa in tailwater areas. Of the original 5.2 million km (3.2 million miles) of rivers in the United States, only 42 high-quality free-flowing rivers greater than 200 km (greater than 124 miles) remain (Benke, 1990). Very few of these rivers occur in the Southeast, and few federal or state programs are being actively pursued to protect these rivers. The federal government owns roughly 30 percent of the land in the United States, which is managed by the U.S. Forest Service, National Park Service, and Bureau of Land Management. Most of this land and inclusive watersheds occur in the western United States or in high-gradient areas (national parks and forests) in the East. Protection of the most biologically significant watersheds is unavailable through federal ownership.
The Wild and Scenic Rivers Act of 1968 provided a means to identify and conserve river reaches and to prohibit federal assistance on water projects that would adversely affect the naturalness of rivers. The legislation, however, did not protect rivers from private development beyond a narrow corridor (Goldfarb, 1988). As of December 1994, 17,175 km (10,672 miles) of river reaches were designated as wild and scenic under the Act (American Rivers, 1994). Of the 150 designated river reaches, only 17 are in the Southeast (Table 13). Arkansas dominates this region with 336 (45 percent) stream km (208.7 miles) under legislative protection, whereas Virginia and Tennessee have no stream reaches in the system. Most designated reaches are in national forests and do not include rivers and watersheds with high molluscan diversity in most urgent need of protection. The bulk of protection has seemingly been directed at streams that already receive federal, state or local protection under other means; i.e., streams of least resistance. American Rivers, the largest national river conservation organization, identified 25 rivers in 1993 considered to be the most endangered and threatened in the United States (American Rivers, 1993). On that list were the Everglades in Florida, St. Marys River in Virginia, Tennessee River in Kentucky, and White River in Arkansas. The Everglades, threatened by water diversion and pollution with agricultural and animal wastes, is home to a diversity of gastropods. The Tennessee and White rivers also have rich molluscan faunas. The Tennessee River, below the Kentucky Dam to its confluence with the Ohio River, is home to threatened and endangered mussels and is a state-designated mussel sanctuary. Hazardous wastes and toxic chemicals emanating from companies in Calvert City, Kentucky have placed the river fauna at risk. The White River is threatened by animal waste effluent, by effluent of wastewater treatment plants from the upper Missouri portion of the basin, and by resultant dissolved oxygen deprivation in the lower river. These organic wastes threaten finfish and shellfish in the reservoirs and free-flowing streams in the White River basin.
Table 13. Components of the National Wild and Scenic Rivers System in the southeastern United States in 1994. |
|||
River |
State |
Administrating Agency |
Total River Kilometers (Miles) |
Sipsey Fork of the West Fork |
AL |
U.S. Forest Service |
98.2 (61.0) |
Big Piney |
AR |
U.S. Forest Service |
72.3 (44.9) |
Buffalo River |
AR |
U.S. Forest Service |
25.3 (15.7) |
Cossatot River |
AR |
U.S. Forest Service Corps of Engineers State of AR |
49.3 (30.6) |
Hurricane Creek |
AR |
U.S. Forest Service |
24.8 (15.4) |
Little Missouri River |
AR |
U.S. Forest Service |
25.1 (15.6) |
Mulberry River |
AR |
U.S. Forest Service |
89.6 (55.7) |
North Sylamore Creek |
AR |
U.S. Forest Service |
23.2 (14.4) |
Richland Creek |
AR |
U.S. Forest Service |
26.4 (16.4) |
Loxahatchee River |
FL |
State of FL |
12.0 (7.5) |
Red River |
KY |
U.S. Forest Service |
31.0 (19.3) |
New River |
NC |
State of NC |
42.4 (26.3) |
Chattooga River |
NC, SC, GA |
U.S. Forest Service |
91.0 (56.5) |
Saline Bayou |
LA |
U.S. Forest Service |
30.4 (18.9) |
Black Creek |
MS |
U.S. Forest Service |
33.6 (20.9) |
Horsepasture River |
NC |
U.S. Forest Service |
6.7 (4.2) |
Obed River |
TN |
National Park Service |
72.3 (44.9) |
Total |
753.6 (468.3) |
||
With such an underfunded effort by regulatory agencies to maintain the biological diversity and integrity of rivers in this region, it is little wonder that the extirpation and extinction of mollusks is occurring at an accelerated pace. The vanguards of river protection have become the national conservation groups such as American Rivers, Izaac Walton League, The Nature Conservancy, and Sierra Club, as well as local organizations such as the Cahaba River Society and others in the Southeast. Citizens organized into local groups under titles such as river keepers, stream and lake watch groups, and friends of various rivers have become the watch dogs of water quality. These groups are making considerable contributions to conservation and protection of water resources and should be encouraged to become more active and vocal in issues that threaten the integrity of aquatic habitats. The designations of outstanding resource waters in states have come most often by local organizations and grass roots support at the county and state level. The shakers and movers of aquatic habitat conservation are those individuals with a long-term vision and vested interest in their living space and quality of life. The 21st century will owe these visionaries a debt of gratitude for their perseverance to sustain islands of aquatic life amid clonal landscapes that have lost their biological exuberance.
The accelerating rate of decline of aquatic mollusks and other faunal groups in aquatic ecosystems of the Southeast is a national tragedy. Federal laws such as the Endangered Species Act, National Environmental Policy Act, Clean Water Act and others have decelerated but not prevented the degradation of habitats and the loss of biological integrity that we chronicle for posterity. Short-sighted and economically suspect projects damaging to mollusk populations, such as the proposed dredging of the Big Sunflower River, Mississippi, continue to appear. Even administrators acknowledge the recurrence of pork barrel projects, initiated to please wealthy constituents of local and national politicians at the expense of federal taxpayers (Bean, 1994). Riverine ecosystems are capital assets that will sustain local communities and economies as long as they are renewable, to provide long-term benefits to generations of residents. Sustainable development must include the wealth and health of the local environment; otherwise, people and businesses will seek greener pastures.
The piecemeal approach to conservation, focused on particular species and habitats, has not been effective. However, there is promise in new initiatives being proposed by federal agencies to address habitat and biodiversity issues on a watershed or ecosystem level. Water quality is inextricably linked to landscape ecology and land-use patterns in the watershed. The new vision of the U.S. Fish and Wildlife Service and National Biological Service is to conserve the nation’s native animal and plant diversity through the perpetuation of dynamic and healthy ecosystems. Ecosystem management will promote the sustainability of ecological functions in watersheds of the Southeast. Thus an ecosystem approach to fish and wildlife conservation will enable natural resource agencies to conserve and restore the structure, function, and natural assemblage of biota in ecosystems, while accommodating sustainable economic development. The long-term viability of healthy ecosystems mandates compatibility between the needs of humans and those of our fellow creatures. As with most aquatic organisms, mollusks living invisibly beneath the water surface do not stir human emotions of endearment or physical affection. The bias towards terrestrial life and its conservation is an ecological malediction (Ryman et al., 1994). In spite of this inherent prejudice, the ecological and technological knowledge of scientists, engineers, and environmental specialists is of sufficient acuity to conserve aquatic species and resolve concerns for competing demands for water, environmentally safe effluent, restoration of damaged habitats, and maintenance of biological diversity and integrity. Implementing a program of ecosystem conservation requires the cooperation of all agencies that share responsibilities for public waters and the biota therein, as well as individuals who reside along waterways that are easily jeopardized.
Riparian landowners are the keystone players in aquatic ecosystem management. Without their interest, concern, and willingness to do what is best for the long-term sustainability of their properties and adjacent streams, holistic management will fail. Therefore, public education and awareness is crucial to establish the network of partnerships necessary to implement conservation in a country that has condoned economic development at any cost. News media lament the destruction of biodiversity in tropical rain forests while neglecting the comparably significant biological diversity in our southeastern rivers. Should we not tend to the needs of our own globally significant aquatic diversity before chastising other nations for their short-sighted mismanagement? We should educate as practitioners, rather than as pedagogues. One of the most respected aquatic malacologists of the early 20th century provided prophetic insight to what was and is a lasting legacy (Ortmann, 1909; page 91): "The destruction of our freshwater fauna forms a chapter of the book on the destruction of our natural resources, a record which is not at all to the credit of the nation."