
"The last word in ignorance is the man who says of an animal or plant: ‘What good is it?’" — Aldo Leopold, A Sand County Almanac
Hum
Most concern by contemporary scientists and conservation groups about biodiversity loss focuses on regions of rich terrestrial diversity such as tropical rain forests. Much less attention is directed at the decline and loss of aquatic species, communities, and ecosystems, whether they are Amazonian, coral reef, or southern Appalachian (Lydeard and Mayden, 1995; Stiassny, 1996).
ans are emotionally biased towards terrestrial biotas and particularly towards those species that are warm-blooded and endearing — the so-called "charismatic megafauna." It is lamentable that this emotional bias is transferred to the intellectual arena by what science is funded and which resources receive protection (Warren et al., 1997). Relatively few resource managers and science leaders are aware of the rich temperate freshwater biodiversity in rivers of the southeastern United States, or of the alarming levels of imperilment of these systems.
North America north of Mexico harbors the richest temperate freshwater fish fauna in the world (Page and Burr, 1991) and therefore is an important global biodiversity resource. This ichthyofauna consists of about 800 species, of which some 490 species occur in the southeastern United States and about 349 inhabit the southern Appalachians. The Etowah River system harbors 91 native fishes (26 percent of the southern Appalachian fish fauna) and 14 introduced species. The southeastern United States is also rich in other aquatic faunas including freshwater mussels and snails (Neves et al., 1997), aquatic insects (Morse et al., 1997), and crayfishes (Taylor et al., 1996). The levels of extinction and imperilment of fishes and mollusks (mussels and snails) in southern states are truly disturbing: about 20 percent of the fish fauna (Williams et al., 1989; Warren and Burr, 1994; Walsh et al., 1995) and over 70 percent of the mollusk fauna (Neves et al., 1997) are in jeopardy. Although less is known about aquatic arthropods, we suspect that similar levels of decline exist in these animals as well. The fact that the arthropod diversity of the southeastern United States is so poorly known and we do not know the extent that the fauna is imperiled exemplifies a general lack of commitment to study of southeastern aquatic resources.
Causes of habitat destruction and aquatic faunal decline are discussed throughout this volume. The ultimate causes are ignorance and greed in the overall way society perceives and exploits the Earth’s finite natural resources. The consequences of this exploitation are exacerbated by human population size and its continued exponential growth. However, there is basis for hope that society will make commitments and take actions necessary to retain most of the existing biodiversity. Humans have clearly advanced in our moral commitment to the natural world (Nash, 1989). Based on the success of nature television programming, more Americans are concerned now than ever before about the intrinsic values of biodiversity.
Relative to biological conservation, the traditional roles of scientists have been as theoreticians with few real data, prognosticators of doom and gloom, or scribes of decline. Conservation science has matured into a focused, multidisciplinary field that incorporates elements of systematics, ecology, population biology, and genetics to study the causes and patterns of biological simplification, and is beginning to offer scientifically-based solutions to restoring damaged biological systems. Many conservation scientists now recognize that communicating only with other scientists or exclusively relying on government agencies to solve onerous and often politically charged environmental problems is simply not working. Furthermore, at the local level, civic leaders and resource managers are often incapacitated by capricious changes in political will. If meaningful changes are going to occur to correct the most blatant abuses of our aquatic resources, scientists must repackage and effectively communicate their esoteric knowledge directly to the public (Tangley, 1994).
In this paper, we use the Etowah River of north Georgia as a conceptual and practical model for restoration of an imperiled southern Appalachian river system. The terrestrial and aquatic components of the Etowah River watershed together compose the Etowah River ecosystem, and we emphasize that the streams in the watershed cannot be considered independently from the lands they drain. Freshwater fishes are primarily used herein to characterize the aquatic health of the river system because our knowledge of fishes is more comprehensive than any other taxonomic group of the southeastern aquatic fauna. However, because of significant imperilment and extirpation levels of mollusks, we also discuss their status in the Etowah River.
The Etowah River is representative of southern Appalachian river systems: it drains southern Appalachian physiography, it has relatively high aquatic diversity, and high fish endemism. It is beleaguered by a litany of environmental threats that are common to southern Appalachian river systems. As a result of habitat decline, the Etowah River system has lost many species and has others placed on state and federal endangered species lists. This contribution synthesizes original data and information from diverse sources. Via this synthesis, we hope to provide critical background information on the status of the Etowah River that will be useful for protection and recovery of this unique ecosystem.
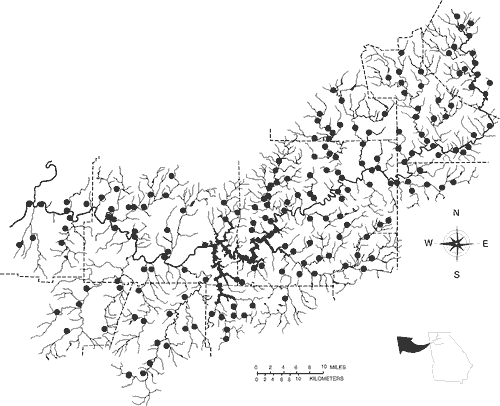
Records of fishes are largely based on about 250 collections made by ourselves and colleagues since 1989, but also include a relatively complete survey of national and regional museum holdings and a summary of literature records. Our initial survey work focused on determining the distribution of the threatened Cherokee darter and later expanded into a general survey of Etowah River fishes. Figure 1 depicts Etowah River collection sites (some recent collection sites are not shown).
Collections were made by seining, backpack shocking, boat shocking, and gill netting. All specimens were retained except for those of the most common or protected species. Most of the specimens collected during our surveys are housed at the Florida Museum of Natural History at the University of Florida (UF). Significant holdings of Etowah River fishes are also at the University of Georgia Museum of Natural History (UGAMNH) and the University of Tennessee at Knoxville (UT). Fish nomenclature is based on a combination of Robins et al. (1991), Mayden et al. (1992), and our interpretation of Coburn and Cavender (1992; page 333) regarding recognition of the minnow genera Ericymba and Hybopsis.
Ecological associations of imperilment for Etowah River fishes are derived from our ongoing study of imperilment patterns of southern Appalachian fishes. We summarized a subset of Etowah fish data from the southern Appalachian fauna to generally compare patterns of imperilment for Etowah River and southern Appalachian fishes. The southern Appalachian analysis is based on a matrix of 349 southern Appalachian fishes (contrasting non-imperiled versus imperiled species) and 46 ecological and zoogeographic attributes similar to a study by Angermeier (1995) for the Virginia fish fauna. Attributes were grouped into related categories, and the set of categories for Etowah River fishes included in this review are range size, vertical orientation in the water column, body size, and habitat size. The matrix for each category consists of species (rows) and attributes (columns).
For definitions of basin, drainage, and system, we use those of Page and Burr (1991). The Etowah River is actually a subsystem of the Coosa River system, but throughout this paper we refer to the Etowah River as a system. The usual definition of an endemic freshwater fish is a species that is restricted to a drainage (Jenkins and Burkhead, 1994), but here we restrict it to a species found only in one river system.
The category range size includes four attributes:
No attempt was made to distinguish between species which have always been highly localized and others with relictual distributions (i.e., those having experienced range constrictions over geologic time).
The category vertical orientation in the water column is divided into two attributes:
Each attribute was averaged across three activities: feeding, spawning, and sheltering.
The category body size is divided into four attributes:
The category habitat size association is represented by five attributes:
Headwater — tiny headwater or first order tributaries, or small springs that are usually less than 2 m (6.6 feet) wide (stream order is a system that characterizes the degree of branching of a stream system; order number increases when two tributaries of the same order join);
Our definition of the imperiled fishes and mussels, with few exceptions, includes species federally listed under the U.S. Endangered Species Act of 1973 as threatened or endangered (U.S. Federal Register, 1994a), Category 2 species (U.S. Federal Register, 1994b), and those listed as special concern, threatened, or endangered by Williams et al. (1989) and Williams et al. (1992b). Until intensive status surveys are made for the lined chub, burrhead shiner, frecklebelly madtom, and freckled darter, we regard their most appropriate status to be Category 2 (versus 3C in U.S. Federal Register, 1994b). Also included as a C2 species is an undescribed darter, Percina sp. cf. P. macrocephala. The populations of this species appear to be disjunct and confined to the upper Coosa River system.
We do not support the recent elimination by the U.S. Fish and Wildlife Service of Category 2 candidates from the Animal Candidate Review for Listing as Threatened or Endangered Species that is periodically published in the U.S. Federal Register. Contrary to the argument by Sayers (1996), we believe the C2 status to be a useful category equivalent to Special Concern status employed by the American Fisheries Society and by many states. In effect, this action orphans declining elements of the fauna from direct federal responsibility until they are in imminent jeopardy and merit listing as threatened or endangered.
Assignment of ecological attributes for species was based on published information. Because of incomplete life-history data on the southern upland fish fauna, matrix cells were scored as either present (1) or absent (0), and no cells were left blank. Body and range size categories had a single attribute score of "1" per species. Habitat size and vertical orientation categories frequently had multiple attributes scored as "1" for each species. For example, in the habitat size category, many species occupy creeks, small rivers, and large rivers, and in the vertical orientation category, some species feed at all depths in the water column. Where data for a species were unavailable, we scored cells based on knowledge of closely related taxa or on the morphology of the species in question. Approximately one-third of the fauna required estimating attribute cell scores. However, based on our knowledge of the fish fauna and experience in compiling an encyclopedic reference summarizing life-history data (Jenkins and Burkhead, 1994), on the whole we believe that our attribute assignments for missing data closely approximate reality. Matrices were summed by columns (attributes) for proportional comparisons of the imperiled and non-imperiled subsets of Etowah River and southern Appalachian fishes.
"We do not inherit the land from our parents — we borrow it from our children." — Native American adage
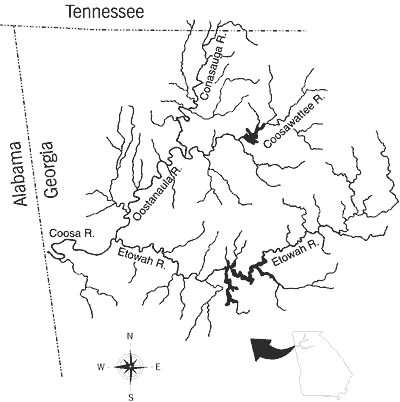
The Etowah River is one of four major headwater tributaries of the upper Coosa River system of the Mobile River drainage. The Etowah River joins the Oostanaula River in Rome, Georgia, to form the Coosa River (Figure 2).
Most of the Coosa River in Alabama is impounded; therefore much of the surviving biodiversity of this system is confined to major tributaries in Alabama and Georgia.
The Etowah River occurs entirely within Georgia; its headwaters originate in the southern boundary of the Blue Ridge and the river flows southwesterly through the Upland subsection of the Piedmont, the Talladega subsection of the Blue Ridge, and the Great Valley subsection of the Valley and Ridge provinces (Wharton, 1978; Figure 3).
County and principal tributary names are depicted in Figure 4. In the center
of the watershed is Allatoona Reservoir, a large 4,800-ha (11,861-acre) hydroelectric
and flood-control impoundment that was completed in 1949 by the U.S. Army Corps
of Engineers (Martin and Hanson, 1966). Allatoona Dam is located in a narrow
gorge in a zone of faults (Cressler et al., 1979) that, prior to impoundment,
probably represented a natural transition area in the character of the river
from rolling upland hills and isolated ridges to the valley floor of the Great
Valley subsection. Geologically, this fault zone roughly delineates a transition
from crystalline metamorphic rocks to sedimentary limestones (Wharton, 1978).
Selected physical attributes of the Etowah River system are as follows: drainage
area = 4,871 km2 (3,027 square miles); drainage area above Allatoona Reservoir
= 1,588 km2 (987 square miles); elevation at mouth = 174 m (571 feet); maximum
elevation = 769 m (2,523 feet); greatest stream order = 6; drainage pattern
= dendritic; main channel length = 265 km (165 miles); impounded channel length
= 51.8 km (32.2 miles); and average gradient = 2.2 m per km (11.6 feet per mile).
Historically, the Etowah watershed was inhabited by Native Americans who occupied
a major village at a site presently known as the Etowah Indian Mound, located
below the Allatoona Dam site (van der Schalie and Parmalee, 1960). Remnants
of Native American fish traps still exist in the main channel of the river above
and below Allatoona Dam (Figure 5).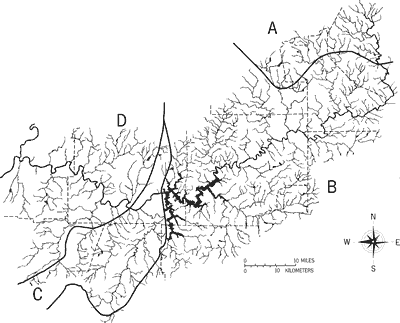
The Etowah River drains portions of 11 counties. Much of the land use in these counties is agricultural, but there are large cities within and adjacent to the watershed, most notably the sprawling Atlanta metropolitan area south of the system (Figure 6). Other large or moderate-sized cities are Rome, located at the mouth of the Etowah River; Cartersville, situated downstream from Allatoona Reservoir on the north side of the river; and Canton, on the south side of the river upstream from Allatoona Reservoir (Figure 6). In decreasing order, Fulton, Cobb, Floyd, and Cherokee are the most urbanized counties in the watershed.
The main channel and most tributaries of the Etowah River are constantly turbid as a result of soil erosion. During normal water levels, water color is typically brown or greenish, but during high water levels the water is orange-red, characteristic of the clay soils of the region. Little is known about the quality of the river prior to 1900. Jordan (1877) characterized a few tributaries of the lower Etowah River as clear with rocky bottoms as compared to the muddy waters of the Ocmulgee River of Georgia. In his autobiography, Jordan (1922) described the main channel of the Etowah River as muddy during a brief collecting trip in 1876. Given the physiography and surface geology of the drainage, there is little doubt that the Etowah River system was historically as clear as the upper Conasauga River, a sister tributary of the Etowah in the upper Coosa River system. By visiting the Conasauga River in northern Murray County, Georgia, or Polk County, Tennessee, one can see clear water conditions that undoubtedly existed in the Etowah River prior to extensive deforestation and land disturbance.
Table 1. Imperiled aquatic species in the Etowah River system. Status categories: C2 = may merit listing, but more data are needed to determine status; T = threatened; E = endangered. Asterisks denote fishes considered extirpated from the river system. |
|||
Common Name |
Scientific Name |
Status |
|
|
|
Federal |
Georgia |
|
Fishes: |
|||
|
Acipenser fulvescens |
C2 |
- |
|
Cyprinella caerulea |
T |
E |
|
Hybopsis lineapunctata |
C2 |
- |
|
Notropis asperifrons |
C2 |
- |
|
Cycleptus sp. cf. C. elongatus |
C2 |
- |
|
Noturus munitus |
C2 |
E |
|
N. nocturnus |
- |
E |
|
Etheostoma ditrema |
C2 |
T |
|
E. etowahae |
E |
T |
|
E. scotti |
T |
T |
|
E. trisella |
C2 |
- |
|
E. sp. cf. E. brevirostrum |
C2 |
T |
|
E. sp. cf. E. brevirostrum |
C2 |
T |
|
Percina antesella |
E |
E |
|
P. brevicauda |
C2 |
- |
|
P. lenticula |
C2 |
E |
|
P. sp. cf. P. macrocephala |
C21 |
- |
Mussels: |
|||
|
Epioblasma metastriata |
E |
E |
|
E. othcaloogensis |
E |
E |
|
Lampsilis altilis |
T |
T |
|
L. perovalis |
T |
- |
|
Lasmigona holstonia |
C2 |
- |
|
Medionidus acutissimus |
T |
T |
|
M. parvulus |
E |
E |
|
Pleurobema decisum |
E |
E |
|
P. georgianum |
E |
E |
|
P. rubellum |
E |
- |
|
P. perovatum |
E |
E |
|
Ptychobranchus greeni |
E |
E |
Snails: |
|||
|
Elimia capillaris |
C2 |
- |
|
E. gerhardti |
C2 |
- |
|
Pleurocera foremani |
C2 |
- |
Insects: |
|||
|
Heterocleon berneri |
C2 |
- |
Totals: |
33 |
19 |
|
1 Status is our recommendation; not listed as such in U.S. Federal Register (1994b). The Alabama shad (Alosa alabamae), a species extirpated from the Etowah River system, may warrant future consideration as a C2 species. |
|||
The Coosa River and its major tributaries, including the Etowah River, may
hold the dubious distinction of having more recent extirpations and extinctions
of aquatic organisms than any other equally-sized river system in the United
States. Neves et al. (1997) document the recent mass extinction of 38 species
of endemic aquatic snails in the Coosa River. We estimate the Etowah River has
more imperiled fishes (17 spp.) and invertebrates (16 spp.) than any other river
system of similar length in the southeastern United States (Table 1). The Conasauga
River has the second highest number of imperiled species (ten fishes and 15
mollusks). The high levels of imperilment in the Etowah and Conasauga rivers
result in large part from the diminution of the high biodiversity of the Coosa
River system caused by extensive habitat loss such that only remnants of formerly
more widespread assemblages survive in fragmented headwater systems. The high
levels of imperilment of the aquatic fauna are not restricted to the relatively
intact portions of the upper Coosa River system; many species are jeopardized
throughout the entire Mobile River drainage (Lydeard and Mayden, 1995).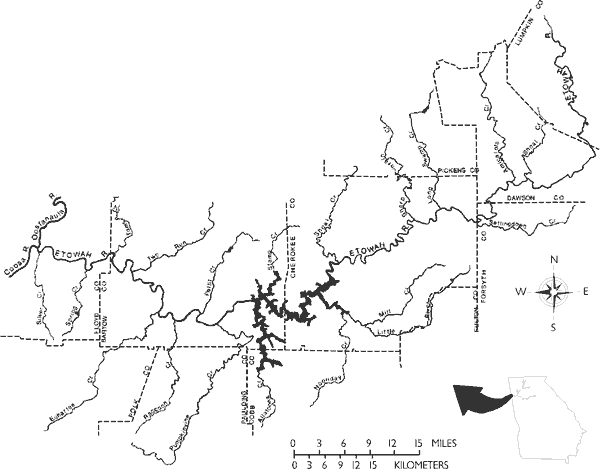
We tabulate 17 fishes as imperiled in the Etowah River: two each threatened or endangered, 12 C2 species, and one species on the Georgia state list but not on federal lists. Twelve Etowah River mussels are on federal lists: eight endangered, three threatened, and one C2 species. The mussel list includes five species (upland combshell, southern acornshell, orange-nacre mucket, Coosa moccasinshell, and ovate clubshell) for which there are no museum records, but that are included based on literature or on probable occurrence given their distribution elsewhere in the Coosa River system. Additionally, three gastropods (snails) and one mayfly in the Etowah River are C2 species (Table 1).
Nine of the 17 imperiled fishes are believed to be extirpated. How much of the imperiled mussel species are extirpated is unknown. Combined, this beleaguered fauna overwhelmingly indicates that the Etowah River system has been and continues to be a system under severe stress and in need of immediate remedial attention.
Our earliest knowledge of the fishes of the Etowah River is from collections made by the eminent ichthyologist David Starr Jordan and students in 1876 (Jordan, 1877, 1878; Jordan and Brayton, 1878). The first list of Etowah River fishes was included in a state list compiled by Dahlberg and Scott (1971). Additions to a list of the Etowah fish fauna, as range extensions or descriptions of new species, were made by Suttkus and Raney (1955), Richards and Knapp (1964), Ramsey and Suttkus (1965), Clemmer and Suttkus (1971), Williams and Etnier (1977), Bryant et al. (1979), Burr and Cashner (1983), Suttkus and Etnier (1991), Wood and Mayden (1993), Suttkus et al. (1994), and Bauer et al. (1995). The list of fishes presented herein is the most comprehensive tally to date of the Etowah River ichthyofauna.
The total historic and present fish fauna of the Etowah River numbers approximately
105 species plus one stocked hybrid (striped bass ´ white bass) (Table 2). Uncertainty
about the exact number of species inhabiting the Etowah River system relates
to the former occurrence of species that may now be extirpated from the system
(see Fish Extirpations), and to the taxonomic status of putative new
taxa. Of the 105 species, 91 are native. Fifteen of the native species are extirpated
from the system, 14 plus one hybrid are nonindigenous, and four are endemic
to the Etowah River (Table 2). We consider this list provisional and likely
to change in the future. The Conasauga River has long been considered to have
the greatest fish and mussel diversity within the upper Coosa River system (Etnier
and Starnes, 1994). Only recently has the Etowah River been identified as having
an equally diverse, but greatly threatened aquatic fauna (Burkhead et al., 1992;
Bauer et al., 1995). We now believe the Etowah River was historically a center
of aquatic biodiversity in the eastern Mobile River drainage.
The most striking characteristic of the Etowah River ichthyofauna is its high level of fish endemism (four species). To compare the number of endemic fishes in the Etowah River system to that of other systems in the Mobile River drainage, we compared the proportion of endemic fishes to total fish species among the Black Warrior, Cahaba, Conasauga, and Etowah rivers (Table 3). In the tally of total species, we did not include nonindigenous species and marine invaders (except for diadromous forms) that are reported to have penetrated the upper Coosa system in western Alabama or Georgia (Dahlberg and Scott, 1971; Boschung, 1992). Sources of data were as follows: Black Warrior, Mettee et al. (1989); Cahaba, Pierson et al. (1989); and our data for the Conasauga and Etowah rivers. The Black Warrior River has more endemics than the Etowah River (six versus four), but the drainage area of the Black Warrior River is nearly four times as large as that of the Etowah River, and the proportional differences of endemics for the Black Warrior versus Etowah (5.1 versus 4.4 percent) are close. The Etowah River is closest in drainage area to the Cahaba River but has twice the number of endemic fishes.
Cryptic Fish Taxa
All the presumed endemic species noted in Table 2 are recently described or recently discovered taxa. The Etowah darter (Figure 7A) was described in 1993 (Wood and Mayden, 1993), and the Cherokee darter (Figure 7C) in 1995 (Bauer et al., 1995). The remaining undescribed endemic fishes (two darters) were discovered by us in 1993 and 1994. Additionally, two undescribed minnows and one undescribed sucker are known or presumed to have occurred in the Etowah River. All these taxa are morphologically cryptic species similar to the nominal taxa with which they have long been placed. The detection of the undescribed darters was based on discovery of subtle but distinct differences between the two forms in male nuptial coloration. Although the study of these putative darter taxa is presently incomplete, we hypothesize that specific differences exist analogous to those of the greenbreast darter group analyzed by Wood and Mayden (1993). Recognition of these putative species is based on a greater level of systematic scrutiny of the Etowah fish fauna than has previously been applied, and parallels recognition of cryptic darter taxa by other ichthyologists in the Southeast (Boschung et al., 1992; Page et al., 1992).
Fish Extirpations
Fifteen fish species, 16 percent of the nativefauna, are considered to be extirpated from the river system (Table 2). A few of the extirpated forms are globally imperiled: lake sturgeon, blue shiner, and coldwater darter. Their extirpation from the Etowah River is not surprising given widespread habitat deterioration. At least two extirpations (American eel and lake sturgeon) were likely caused by multiple impoundments of the Coosa River system, including Allatoona Reservoir. However, the demise of much of the missing fauna is enigmatic because many of these species are generally regarded as tolerant or persistent species and occur in other degraded rivers. While we have no recent records for these species, we find it difficult to believe that some of them actually are extirpated (e.g., chain pickerel, lined chub, creek chubsucker, and blackspotted topminnow). Perhaps provisional listing here will promote their "rediscovery." Two suckers not included as extirpated are puzzling. The spotted sucker, a relatively tolerant and usually common species, has not been collected by us although it is known from the Etowah River (Beisser, 1989; year of capture not specified). Unknown from the Etowah River is the sometimes elusive river redhorse. We captured the river redhorse in the lower Oostanaula River and suspect that a low-density population persists in the lower Etowah River. Documentation for the extirpated fish fauna, as presently conceived, is as follows:
Lake sturgeon — This species is known from the Coosa and Oostanaula rivers (Boschung, 1992). One of us (BJF) heard an anecdotal report of someone catching a 1.5-m (4.9-feet) specimen with a pitchfork below the spillway dam in Cartersville in the late 1940s. The species is probably extirpated from Georgia and Alabama (Boschung, 1992).
American eel — Jordan and Brayton (1878) listed the American eel as "abundant" in the Alabama River system. A landowner informed one of us (BJF) that eels used to be common in the Etowah River above Allatoona Reservoir.
Alabama shad — Not known from the Etowah River. Boschung (1992) reported it from the Coosa River below Jordan Reservoir. We suspect that prior to multiple impoundments of the Coosa River, the Alabama shad probably occurred in the lower Etowah and Oostanaula rivers. The closely related American shad was known to penetrate the Blue Ridge in the James River in Virginia prior to construction of spillway dams at and above Richmond (Jenkins and Burkhead, 1994).
|
|
|
|
|
|
|
|
Figure 7. Cryptic species recently described from the Etowah River: Top = Etowah darter, Etheostoma etowahae (male, 47 mm standard length, Etowah River, Lumpkin County, GA), similar to second = greenbreast darter, E. jordani (male, 58 mm standard length, Shoal Creek, Cleburne County, AL); third = Cherokee darter, E. scotti (male, 55 mm standard length, Shoal Creek, Dawson County, GA), similar to fourth = Coosa darter, E. coosae (male, 58 mm standard length, Moseley Spring,Chattooga County, GA) (photographs by N.M. Burkhead). |
Blue shiner — One lot of this species at Cornell University (CU 1488) lists the following locality data: Etowah River, Jordan, October 1876. However, Jordan (1877) and Jordan and Brayton (1878) did not report the blue shiner from the Etowah River. Gibbs (1955) erroneously listed records of the blue shiner from the Little River in Cherokee County, Georgia; these were actually from the Little River in Cherokee County, Alabama. The blue shiner was historically distributed in the Oostanaula and Coosa rivers in the Valley and Ridge province (Gilbert et al., 1980). We believe it formerly occurred in the Etowah River system.
Lined chub — Jordan (1877) reported it as "abundant in all tributaries of the Etowah" In their list of material examined, Clemmer and Suttkus (1971) reported two localities for the lined chub above Allatoona Dam, and in their distribution map, the authors plotted another record below Allatoona Dam.
Burrhead shiner — Suttkus and Raney (1955) examined specimens at the United States National Museum collected by Jordan from the Etowah River (USNM 164968 and USNM 164969, one specimen each). These appear to be the last burrhead shiners collected from the Etowah River system.
"Blue sucker" (undescribed Mobile drainage form) — This interesting large-river sucker is not known from the Etowah River system. However, there were no extensive main-channel collections made in the upper Coosa, or lower Etowah and Oostanaula rivers prior to extensive impoundment of the Coosa River. Historically, the blue sucker probably penetrated the upper Coosa River system, including the lower reaches of the Etowah and Oostanaula rivers.
Creek chubsucker — Jordan (1877) reported it from the Etowah River, but later called it the lake chubsucker (Jordan, 1878). Because the mouth of the Etowah River is a significant distance above the Fall Line, we speculate that Jordan’s record was based on creek chubsuckers.
Freckled madtom — In the Etowah River it is known from only one specimen collected in 1962 (UGAMNH 814). The freckled madtom is a widespread species within the Mississippi Embayment (Rhode, 1980). The Etowah River record represents the most upstream collection of the species in the Coosa River system.
Chain pickerel — Jordan (1877) reported it as abundant in tributary ponds of the lower Etowah River system. To our knowledge, no pickerels have since been captured from the Etowah River, thus indicating another enigmatic disappearance.
Blackspotted topminnow — There are no records of this species from the Etowah River. However, it is known from Big Dry Creek (Freeman, 1983), an Oostanaula tributary adjacent to the Etowah River. We consider it likely that the blackspotted topminnow historically resided in the Etowah River.
Coldwater darter — Jordan (1877) misidentified this species as "Boleichthys elegans" (Ramsey and Suttkus, 1965), stating that it was most common in Dykes Pond near Rome. Dykes Creek, presumably where Dykes Pond was located, presently contains a depauperate fish fauna, and no coldwater darters have been recently collected there. Although no other records of coldwater darters are known from the Etowah River, there are several large spring-fed tributaries in the Valley and Ridge portion of the system that may have supported coldwater darter populations.
Trispot darter — There are no records of this species from the Etowah River. It is known from the Valley and Ridge province of the Conasauga and Oostanaula rivers (Freeman, 1983), and we consider it very likely that the trispot darter formerly existed in the lower Etowah River in the same province.
Coal darter — This recently described darter (Suttkus et al., 1994) is not known from the Etowah River. The coal darter is a large-river species known from the main channel of the Coosa River above the Fall Line, and, like the undescribed "blue sucker," we presume it occurred historically in the lower Oostanaula and Etowah rivers.
River darter — There are no Etowah River records of this large-river species. Stiles and Etnier (1971) reported it from the Conasauga River far upstream from the mouth of the Etowah River; therefore, we conclude it formerly occurred in the lower main channel of the Etowah River.
Table 2. Fishes of the Etowah River system. Status symbols: N = native; PN = probably native; I = introduced (i.e., nonindigenous); PI = possibly introduced; EX = extirpated from system; E = exclusively endemic to the Etowah River system. |
||
Family Name (No. Species) |
Common Name |
Status |
|
||
Family Petromyzontidae (3 spp.) |
Lampreys |
|
|
chestnut lamprey |
N |
|
southern brook lamprey |
N |
|
least brook lamprey |
N |
Family Acipenseridae (1 sp.) |
Sturgeons |
|
|
lake sturgeon |
N, EX |
Family Lepisosteidae (1 sp.) |
Gars |
|
|
longnose gar |
N |
Family Hiodontidae (1 sp.) |
Mooneyes |
|
|
mooneye |
N |
Family Anguillidae (1 sp.) |
Freshwater eels |
|
|
American eel |
N, EX |
Family Clupeidae (3 spp.) |
Herrings |
|
|
Alabama shad |
PN, EX |
|
gizzard shad |
N |
|
threadfin shad |
I |
Family Cyprinidae (31 spp.) |
Minnows |
|
|
largescale stoneroller |
N |
|
bluefin stoneroller |
N |
|
grass carp |
I |
|
blue shiner |
N, EX |
|
Alabama shiner |
N |
|
red shiner |
I |
|
tricolor shiner |
N |
|
blacktail shiner |
N |
|
common carp |
I |
|
silverjaw minnow |
PN1 |
|
lined chub |
N, EX? |
|
undescribed chub |
PN1 |
|
striped shiner |
N |
|
bandfin shiner |
PN1 |
|
mountain shiner |
N |
|
undescribed chub |
N |
|
silver chub |
N |
|
bluehead chub |
N |
|
river chub |
PI |
|
golden shiner |
N |
|
burrhead shiner |
N, EX? |
|
rainbow shiner |
N |
|
longnose shiner |
PN1 |
|
yellowfin shiner |
PI |
|
silverstripe shiner |
N |
|
Coosa shiner |
N |
|
riffle minnow |
N |
|
bluntnose minnow |
I |
|
bullhead minnow |
N |
|
blacknose dace |
N |
|
creek chub |
N |
Family Catostomidae (9 spp.) |
Suckers |
|
|
ndescribed "blue sucker |
PN, EX |
|
creek chubsucker |
N, EX? |
|
Alabama hog sucker |
N |
|
smallmouth buffalo |
N |
|
spotted sucker |
N |
|
river redhorse |
N |
|
black redhorse |
N |
|
golden redhorse |
N |
|
blacktail redhorse |
N |
Family Ictaluridae (10 spp.) |
Bullhead catfishes |
|
|
snail bullhead |
PN1 |
|
black bullhead |
N |
|
yellow bullhead |
N |
|
brown bullhead |
N |
|
blue catfish |
N |
|
channel catfish |
N |
|
speckled madtom |
N |
|
frecklebelly madtom |
N |
|
freckled madtom |
N, EX? |
|
flathead catfish |
N |
Family Esocidae (1 sp.) |
Pikes |
|
|
chain pickerel |
N, EX? |
Family Salmonidae (3 spp.) |
Trouts |
|
|
rainbow trout |
I |
|
brown trout |
I |
|
brook trout |
I |
Family Fundulidae (2 spp.) |
Killifishes |
|
|
blackspotted topminnow |
PN, EX? |
|
southern studfish |
N |
Family Poeciliidae (1 sp.) |
Livebearers |
|
|
eastern mosquitofish |
N |
Family Cottidae (2 spp.) |
Sculpins |
|
|
mottled sculpin |
N |
|
banded sculpin |
N |
Family Moronidae (3 spp.) |
Temperate basses |
|
|
white bass |
I |
|
yellow bass |
I |
|
striped bass |
I |
|
hybrid |
I |
Family Centrarchidae (13 spp.) |
Sunfishes |
|
|
shadow bass |
N |
|
redbreast sunfish |
I |
|
green sunfish |
N |
|
warmouth |
N |
|
bluegill |
N |
|
longear sunfish |
N |
|
redear sunfish |
N |
|
redspotted sunfish |
N |
|
redeye bass |
N |
|
spotted bass |
N |
|
largemouth bass |
N |
|
white crappie |
N |
|
black crappie |
N |
Family Percidae (19 spp.) |
Perches |
|
|
Etowah undescribed darter |
E |
|
Amicalola undescribed darter |
E |
|
Coosa darter |
N |
|
coldwater darter |
N, EX |
|
Etowah darter |
E |
|
greenbreast darter |
N |
|
rock darter |
N |
|
Cherokee darter |
E |
|
speckled darter |
N |
|
trispot darter |
PN, EX |
|
undescribed logperch |
N |
|
undescribed darter |
N |
|
amber darter |
N |
|
coal darter |
PN, EX |
|
freckled darter |
N |
|
blackbanded darter |
N |
|
bronze darter |
N |
|
river darter |
PN, EX |
|
walleye |
N |
Family Sciaenidae (1 sp.) |
Drums |
|
|
freshwater drum |
N |
1 We tentatively regard the occurence of these species in the Etowah River to be the result of stream capture with the Chattahoochee River system fide Suttkus and Boschung (1990; page 61). |
||
Understanding shared biological patterns among declining and extinction-prone
species is of strong interest to ecologists (Terborgh, 1974; Willis, 1974; Diamond,
1984; Angermeier, 1995; Parent and Schriml, 1995). For rheophilic freshwater
fishes, life-history and ecological attributes such as range size, body size,
habitat association, and vertical orientation in the water column are attributes
for intuitively investigating correlates of imperilment. Herein we generally
compare these attributes between the non-imperiled and imperiled fishes in southern
Appalachia and the Etowah River. The results of the comparisons are discussed
by category below. These comparisons do not establish causal mechanisms but
they do allow possible inference for further lines of investigation. The results
also contribute to a growing body of evidence that patterns of rarity and extinction
are not isolated, random phenomena (Angermeier, 1995; Parent and Schriml, 1995).
Lastly, understanding that associations of imperilment span shared suites of
ecological adaptations in multiple, evolutionarily diverse families underscores
the concept that single-species management is biologically inappropriate and
fiscally wasteful.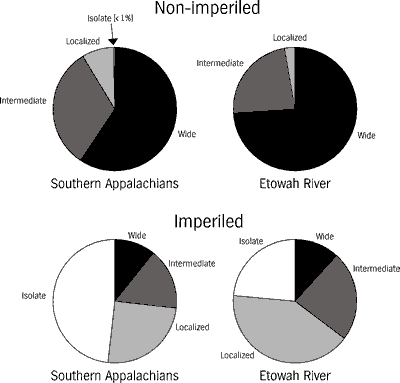
We consider range size to be the most important correlate of imperilment. It is axiomatic that fishes with small ranges are generally more vulnerable to threats, because even small losses of habitat can be proportionally more serious than similar reductions in species with larger ranges (Moyle and Williams, 1990; Burkhead and Jenkins, 1991; Etnier and Starnes, 1991; Angermeier, 1995). This premise is independent of population density, although it is obvious that, of two species with similar small ranges, the species with the lowest population is potentially more vulnerable. Most non-imperiled species of the southern Appalachians and the Etowah River are wide- and intermediate-ranging, whereas most imperiled species are localized or geographically isolated (Figure 8). Proportional differences of localized and isolated range sizes between the non-imperiled and imperiled faunas are considerable. The species with the most restricted distributions in the Etowah River are the amber and the Etowah darters (federally endangered), and two undescribed darters (Etheostoma spp. cf. E. brevirostrum) that are Category 2 species (Table 1). The latter three species are exclusively endemic to the Etowah River. All of the environmentally degrading factors discussed below (see Threats to the System) can cause reductions in range size.
Benthic specialization is an important correlate of imperilment. Obligate benthic species — those that spawn, feed, and shelter on the stream bottom — compose a substantial fraction of the Etowah’s imperiled fish fauna. Obligate benthic specialization occurs throughout the southern Appalachian and Etowah River fish faunas. The ratio of imperiled benthic fishes to imperiled non-benthic fishes is about 5:1, as opposed to about 2:1 for the same ratio among non-imperiled species in both the Etowah River and southern Appalachian faunas (Figure 9). Of the imperiled fishes in Table 1, 80 percent are obligate benthic species and the remaining ones are associated with the substrate by one or more critical life-history activities (spawning, feeding, or sheltering). The primary form of degradation and destruction of benthic habitats is elevated sedimentation, and the present effects of sedimentation may be heightened by pollutants bound to sediments (see Threats to the System).
Table 3. Comparison of areas, species richness, and levels of endemism of four river systems of the Mobile River drainage. Data for native species (excluding nonindigenous and marine invading species) are from Mettee et al. (1989) and Pierson et al. (1989) for the Black Warrior and Cahaba river systems, and our unpublished data for the Conasauga and Etowah river systems. |
|||
River System |
Drainage Area |
Species:Endemics |
Percent Endemic |
Black Warrior |
16,255 km2 (6,274 sq. miles) |
118:6 |
5.1 |
Cahaba |
4,727 km2 (1,825 sq. miles) |
127:2 |
1.6 |
Conasauga |
1,883 km2 (727 sq. miles) |
75:2 |
2.7 |
Etowah |
4,871 km2 (1,880 sq. miles) |
91:4 |
4.4 |
Small body size is associated with imperilment in aquatic and terrestrial vertebrates (Diamond, 1984; Angermeier, 1995). For fishes this is partially because most eastern North American species are less than 150 mm (6 inches) in total length. However, a substantially greater proportion of very small- and small-sized species are imperiled versus medium- and large-sized species (Figure 10). Small body size in fishes is correlated with several other ecological attributes associated with imperilment: low intrinsic dispersal capabilities, short longevity, benthic specialization, and generally reduced reproductive potential. Fifteen of 17 imperiled fishes (Table 1) are very small- and small-sized species. The lake sturgeon and the undescribed "blue sucker" are the only imperiled large-sized and medium large-sized species (that formerly occurred) in the Etowah River.
Because the southern upland fish fauna evolved in, and is primarily associated
with lotic habitats, we examined flowing-water habitat associations of the imperiled
and non-imperiled faunas to determine if differences exist. Prior to our analysis,
we speculated that most of the imperiled species would be associated with headwaters,
small creeks, and large rivers because of the high degree of degradation of
those fluvial water bodies by agriculture and impoundment. Most species in the
southern Appalachians and in the Etowah River occur in creeks and small rivers,
habitat sizes that probably represent most of the total habitat area available.
We found no large differences between frequency distributions of the non-imperiled
and imperiled species (Figure 11). While it is impossible to state that there
is an equal sampling effort across all habitat sizes, particularly with respect
to large rivers, repeated sampling by multiple methods has yielded the best
data available. We further realize that as imperiled species become rarer, the
probability of detecting them significantly declines (Etnier, 1994). Given these
constraints on the best data available, we feel these results imply that habitat
degradation and destruction has been relatively ubiquitous across fluvial habitats;
all sizes of habitats — small headwater creeks to large rivers — have been despoiled
to some degree.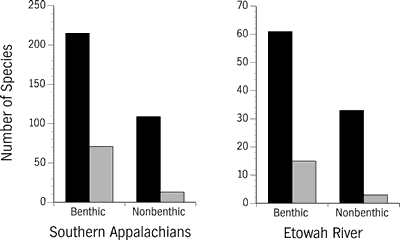
Like temperate freshwater fishes, freshwater mussels or clams (family Unionidae) are most diverse in the southeastern United States. Of the 297 freshwater mussel taxa recognized (281 species and 16 subspecies) in the United States and Canada (Turgeon et al., 1988), about 90 percent occur in the southeastern United States. About 17 percent (51 species) of this mussel fauna is known from the Etowah River system, where species historically occupied diverse habitats ranging from upland creeks to the main channel of the river.
Adult unionids range in size from 4 cm to more than 25 cm (1.6 inches to greater than 9.8 inches) and the largest may weigh 1 kg (2.2 pounds). Mussels are among the longest lived freshwater invertebrates; many species may live 20 to 40 years and a few species live more than 50 years. Freshwater unionids have a unique relationship with fishes in that about 98 percent of all species require a fish host to complete their life cycles (Fuller, 1974; Hoggarth, 1992).
Mussels were historically common throughout freshwater ecosystems of the southeastern
United States. Mussel declines during the past century parallel those of other
freshwater organisms, notably snails, and fishes. Williams et al. (1992b) reported
213 species, about 72 percent of the United States mussel fauna, as endangered,
threatened or of special concern, and 21 species (7 percent) as possibly extinct.
The demise of this widespread and diverse fauna is primarily the result of habitat
destruction and deteriorated water quality of southern rivers.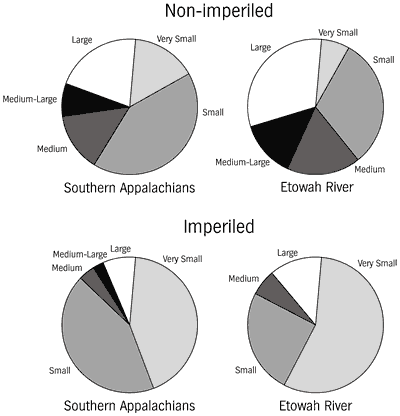
Our provisional list (Table 4) of mussels thought to have historically existed in the Etowah River is based on records from archeological digs, early collections, and inference from known mussel distributions throughout the remainder of the upper Coosa River system. Mussels were collected in the Etowah River watershed for centuries by Native Americans who used them for a variety of purposes, including food, tools, and jewelry. Mussels excavated from middens at the Etowah Mound Site in Bartow County were analyzed by van der Schalie and Parmalee (1960). Mussel collectors sampled the Etowah River in the early to mid-1800s and most of the shells were shipped to wealthy conchologists who maintained them in their personal collections or donated them to natural history museums.
The present outlook of the mussel fauna of the Etowah River is bleak. Burkhead et al. (1992) estimated that as many as 65 percent of the species may have been extirpated from the system. Mussels are susceptible to many pollutants and most species cannot survive in reservoirs (Fuller, 1974; Williams et al., 1992a). Decline of the Etowah River mussel fauna may have begun around the time of the first gold rush in the United States, in northern Georgia. Gold miners used mercury to coalesce gold fines. Mercury contamination in the Etowah River alluvial floodplain has been dated to 1830 in core sediment samples (Leigh, 1994).
Without a thorough and systematic survey for mussels we cannot know what percentage of the Etowah River mussel fauna survives. In over 200 fish collections that we have made since 1989, we have only observed valves of three dead native mussels. Our efforts to detect mussels — while certainly not a survey — have not been casual either. We have intentionally searched river banks while collecting and canoeing, and have walked islands (particularly where we camped) looking for valves in places where they might normally be observed.
One of the most common patterns associated with extirpation is the fragmentation
of a species’ range into small, isolated subpopulations within the larger geographic
area occupied by the species. Habitat and population fragmentation and the scales
on which it can occur are summarized by Harris (1984) and Meffe and Carroll
(1994). Fragmentation results from localized environmental degradation and exists
at all spatial scales. Widespread losses of aquatic habitats in the southern
Appalachians result largely from major impoundments, deforestation, urbanization,
and chronic pollution, including sedimentation. Smaller-scale fragmentation
occurs from scattered, localized habitat perturbations associated with a variety
of anthropogenic activities such as small water supply or farm pond impoundments,
isolated residential developments, riparian clearing, landfills, site-specific
sedimentation, agricultural runoff, and urban sprawl along transportation routes
near streams. While large-scale projects are typically evaluated for potential
environmental impacts, many small-scale projects do not receive such attention.
In the long run, extensive fragmentation by piecemeal habitat loss may actually
harm aquatic species as much or more than large-scale, often controversial projects.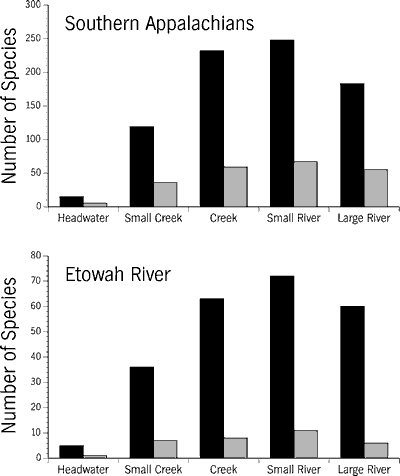
The biological consequences of population fragmentation involve genetic isolation and demographic effects. Genetic isolation can diminish population fitness (Soulé, 1980; Carson, 1983). However, even minimal emigration of a few individuals between insularized populations can maintain at least short-term population heterozygosity (Soulé, 1980). Emigration across relatively permanent barriers such as major dams can be considered "sweepstakes" dispersal, i.e., possible but not likely (Chesser, 1983), while intermittent dispersal across smaller stretches of degraded stream habitat may bolster insular heterozygosity. A pervasive problem in the Etowah River system, and other southern Appalachian rivers, is that ever-increasing habitat loss continually decreases or eliminates temporal opportunities for fishes to disperse across degraded stream reaches. The efficacy of degraded stream habitats contributing to isolation of fish populations has been genetically demonstrated by reduced allele and genotype frequencies in populations of the spotfin shiner (Cyprinella spiloptera) separated by polluted stream reaches (Gillespie and Guttman, 1993), and morphologically demonstrated by the distinctiveness of disjoined populations in the California roach (Hesperoleucas symmetricus) (Brown et al., 1992).
Presently, we know little about the genetic consequences of extensive population fragmentation in southeastern fishes. In the Etowah River system, the distribution pattern of the threatened Cherokee darter suggests severe levels of insularization which warrant further investigation (Figure 12). Bauer et al. (1995) found significant differences in seven out of ten meristic characters between Cherokee darters inhabiting northern and southern tributaries of the Etowah River, suggesting that the degraded main channel of the river was acting as a barrier to dispersal of this tributary species. Barriers creating insularization in the Etowah River have existed for over 100 years as a main-channel spillway dam in Cartersville (Hall and Hall, 1921), and as severe sedimentation and pollution episodes dating to 1830 (Leigh, 1994).
Population responses to habitat fragmentation may contribute to extirpation and extinction more directly than genetic factors (Lande, 1988). Habitat fragmentation directly impacts population dynamics through changes in population size, Allee effects (density-triggered phenomena such as mass emigration or reduction of overall reproductive rates), changes in population structure, decline or extirpation of rare species and habitat specialists, and reduced ability of populations to respond to environmental stochasticity (Shaffer, 1981; Brown, 1984; Lande, 1988; Pimm et al., 1988).
In the Etowah River we have found anomalous distribution gaps of several fish species (e.g., rainbow shiner, Cherokee darter, and rock darter), constricted ranges (e.g., frecklebelly madtom), widespread extirpations (amber and Cherokee darters), and the persistence of a distinct guild of tolerant species in many areas throughout the watershed. A vexing aspect of habitat fragmentation is that rare species may require larger reaches of suitable habitat over time than they actually use at a given time. Freeman and Freeman (1994) discovered that the endangered amber darter, utilized only 40 percent of available suitable habitat during their study. These authors hypothesized that long-term population viability may depend on availability of the additional habitat to support populations during periods of random environmental variation, such as during droughts.
"Large numbers of feathers, balls of fat approximately one inch in diameter, and chicken parts (wings and feathers) were floating downstream." — Georgia Water Quality Control Board (1970)
All modern biologists studying riverine systems or their faunas in the southeastern United States are working on systems that have a history of episodic degradation that transcends several human generations. Sometimes certain southern streams are described as "pristine," but in most cases the modern application of this word is a misnomer.
Table 4. Provisional list of 51 native freshwater mussels (family Unionidae) from the Etowah River system based on: L = literature (van der Schalie and Parmalee, 1960; Hurd, 1974); M = museum records; and P = probable occurrence in the Etowah Drainage based on the species' distribution in the Coosa River system. Other symbols: I = imperiled (endangered, threatened, or special concern as treated by Williams et al., 1992b); E = federally endangered; T = federally threatened. |
||
Scientific Name |
Common Name |
Source & Status |
|
roundlake |
L, M |
|
rayed creekshell |
I, M |
|
butterfly |
I, L |
|
Alabama spike |
I, L, M |
|
delicate spike |
I, L, M |
|
elephant-ear |
L, M |
|
upland combshell |
E, L |
|
southern acornshell |
E, P |
|
Gulf pigtoe |
L |
|
ebonyshell |
L, M |
|
fine-lined pocketbook |
T, L, M |
|
southern pocketbook |
I, L, M |
|
orange-nacre mucket |
T, P |
|
southern fatmucket |
L, M |
|
Alabama heelsplitter |
I, P |
|
Tennessee heelsplitter |
I, L, M |
|
fragile papershell |
L, M |
|
black sandshell |
I, L |
|
Alabama moccasinshell |
T, L, M |
|
Coosa moccasinshell |
E, L |
|
washboard |
L |
|
threehorn wartyback |
L, M |
|
Alabama hickorynut |
I, L |
|
highnut |
I, L, M |
|
painted clubshell |
I, M |
|
southern clubshell |
E, L, M |
|
southern pigtoe |
E, L, M |
|
Georgia pigtoe |
I, L, M |
|
Alabama pigtoe |
L, M |
|
Coosa pigtoe |
I, L |
|
longnut |
I, L, M |
|
ovate clubshell |
E, P |
|
Warrior pigtoe |
I, L, M |
|
Alabama clubshell |
I, L, M |
|
bleufer |
L |
|
triangular kidneyshell |
E, L, M |
|
giant floater |
P |
|
Alabama orb |
I, L, M |
|
monkeyface |
L |
|
ridged mapleleaf |
I, L, M |
|
Alabama creekmussel |
I , L, M |
|
southern creekmussel |
I, L |
|
lilliput |
L, M |
|
pistolgrip |
L, M |
|
fawnsfoot |
P |
|
pondhorn |
L, M |
|
paper pondshell |
P |
|
little spectaclecase |
L, M |
|
Alabama rainbow |
I, L, M |
|
Coosa creekshell |
I, L, M |
|
southern rainbow |
L, M |
The major threats to southern Appalachian rivers are impoundments, sedimentation, harmful agricultural practices, urbanization, and pollution. All of these degrading forces occur to some extent throughout the Etowah River watershed, and these anthropogenic impacts have been repeatedly identified as major causes of aquatic faunal decline and extinction throughout North America (Williams, 1981; Ono et al., 1983; Miller et al., 1989; Williams et al., 1989; Burkhead and Jenkins, 1991; Etnier and Starnes, 1991; Moyle and Leidy, 1992; Warren and Burr, 1994). Below we present data and observations of the effects of impoundment and sedimentation on the Etowah River and a less detailed discussion on the effects of pollution and urbanization.
The present assemblage of drainages, environments, and associated faunas of southern Appalachia began to assume their pre-European colonization configuration after the last glacial retreat at the beginning of the Holocene (10,000 years before present; Hackney and Adams, 1992). Major anthropogenic modifications to riverine habitats began with the arrival of European immigrants. The earliest alterations of North American rivers by European descendants were primarily canals and low-head spillway dams (Palmer, 1986). However, in the years spanning the 1930s to the late 1970s, approximately 144 large dams were constructed in the southeastern United States. Today, 98 percent of all southeastern rivers are blocked by at least one major dam, and some major rivers are nearly or completely impounded (Hackney and Adams, 1992; Soballe et al., 1992). Most large impoundments in the southern Appalachians are hydroelectric-flood control facilities, or are associated with hydrogeneration as pump-storage impoundments. Hydrogenerated electricity only produces about two to three percent of the energy used during peaking power consumption. These colossal edifices are clearly major landscape-scale causes of aquatic faunal decline, fragmentation, and extirpation in southern Appalachia, including the Etowah River. In addition to large impoundments, there are over a million farm pond impoundments on first- and second-order tributaries in the Southeast (Menzel and Cooper, 1992). Negative ecological effects vary between large and small impoundments as discussed below.
Large Impoundments
Large impoundments are not benign replacements of river sections with lakes. Rather, they are hybrid environments (Soballe et al., 1992) that lack the productive littoral zone of natural lakes and the mosaic of riffle-run-pool habitats characteristic of upland streams. Impoundments dramatically alter habitat and reduce biodiversity in the inundated river portion. Hydrogeneration creates episodic fluctuations in downstream flow levels, resulting in dramatic physicochemical changes of the river environment. These negative effects may extend for many kilometers below a large dam. The main deleterious effects of large impoundments include the following: 1) loss of system connectivity; 2) alteration or elimination of natural flood cycles or natural low-water periods; 3) concentration of pollutants and sediments deposited in impounded reaches; 4) providing focal points for introductions of nonindigenous fishes; 5) alteration of nutrient cycling and natural trophic webs; 6) thermal alteration of the river below the dam by hypolimnotic water release; 7) bank destabilization and scouring or armoring (compacting and hardening to a crust-like layer) of downstream substrates; 8) truncation and isolation of tributaries entering the reservoir; and 9) changes in physicochemical parameters below the dam such as daily or seasonal reductions in dissolved oxygen (DO) (U.S. National Research Council, 1992; pages 200-201; Stanford and Ward, 1992, Table 5.1; Yeager, 1994).
Allatoona Reservoir has multiple negative effects on many species of native
fishes and aquatic invertebrates. Besides permanently eliminating significant
segments of the free-flowing Etowah River (ca. 52 river km; about 32.3 river
miles) and lower portions of impounded tributaries, the reservoir has been an
introduction point for several nonindigenous fishes (Beisser, 1989).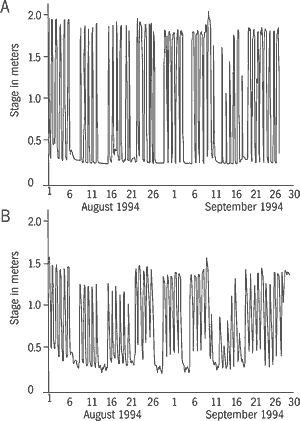
Water release from the dam apparently does not dramatically alter the natural temperature regimes of the river (Stokes et al., 1986). Water quality immediately below the dam was characterized as moderately polluted in 1969, based on low DO concentrations and reduced macroinvertebrate abundance and diversity (Georgia Water Quality Control Board, 1970). While sampling fish in early September 1993 we recorded a low DO of 2.7 parts per million (ppm) and detected the distinct odor of hydrogen sulfide. A persistent benthic boundary layer, high in hydrogen sulfide, occurs in deeper sections of Allatoona Reservoir (G. S. Beisser, Georgia Department of Natural Resources, pers. comm.). Water-level fluctuations in the Etowah River below the dam vary seasonally, weekly, and daily based on hydrogeneration demands. Figure 13 illustrates daily and weekly variations in discharge at a site just below the dam and at a site in the lowermost river during August and September 1994. Weekend reduction of discharges to base flows are evident. Daily fluctuations during these months were approximately 1.5 m (4.9 feet) at the upstream gauge and 1 m (3.3 feet) at the downstream gauge. These daily discharge variations create a highly unstable environment. The fluctuating water levels and associated velocities have scoured and armored the substrate throughout the upper one-third of the river below the dam. Large gravel, rubble, and small boulders of riffles are generally embedded in a matrix of sand and clay, eliminating most interstitial habitat available for macroinvertebrates.
Allatoona Reservoir seriously impacts the main-channel fish fauna below the dam (Figure 14). Only five species, all uncommon, were sampled immediately below the dam: one nonindigenous species (common carp) and four centrarchids. The numbers of fishes collected at sites downstream from the dam were strongly negatively correlated with proximity to the dam. Diversity of the ichthyofauna does not reach a downstream maximum until 64 river km (40 miles) below the dam.
The present downstream fauna probably differs substantially from the fauna
that occurred historically in the vicinity of the dam site, and it is clearly
unlike the main-channel fauna above the dam. The fish community below the dam
is dominated by suckers (Catostomidae), large catfishes (Ictaluridae, excluding
madtoms), and sunfishes (Centrarchidae), and there are notably fewer species
of minnows (Cyprinidae) and darters (Percidae) than in the Etowah River upstream
of Allatoona Reservoir. Nonindigenous species occurring only below the dam are
red shiner and grass carp. Prior to construction of the dam and associated changes,
the total fauna of the lower Etowah River probably exceeded our estimated number
of cumulative species by as much as 30 percent. The extirpated fauna probably
included lake sturgeon, American eel, Alabama shad, undescribed Mobile "blue
sucker," coal darter, river darter, and species surviving elsewhere in
the system such as largescale stoneroller, Alabama shiner, silver chub, bluehead
chub, riffle minnow, speckled madtom, frecklebelly madtom, greenbreast darter,
rock darter, amber darter, and freckled darter.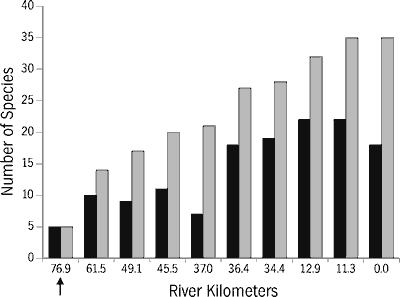
Changes in the fish community structure in flow-regulated sections of the Tallapoosa River were reported by Kinsolving and Bain (1993) and Travnichek and Maceina (1994). Both studies presented evidence of faunal recovery and did not detect the extirpation level or degree of community replacement that we have observed in the Etowah River. The community shifts in the lower Etowah river may be partially due to increased large-river habitat and associated riverine species, e.g., mooneye, smallmouth buffalo, and blue catfish. We suspect that the increase in faunal composition in the lower Etowah River is explained by persistence or recolonization by a guild of relatively tolerant species — the macrohabitat generalists of Kinsolving and Bain (1993). Because our data are qualitative (presence or absence of species), we cannot document any shifts in biomass, but we have observed low abundance of most species in the main channel 30 to 40 km (18.6 to 25 miles) below the dam. Other factors that may also contribute to an increase in the total number of species in lower river segments are improved water quality by dilution from tributaries and progressive amelioration of fluctuating water levels. The fact that the Tallapoosa fish fauna shows greater recovery at similar distances below large hydropower dams than is evident in the lower Etowah, despite similar faunas in the two rivers (Table 2; Williams, 1965), suggests that additional factors may be causing faunal depression in the lower Etowah River. Diminution of the Etowah River fauna below Allatoona Dam may be the collective result of hydrogeneration, pollution, and sedimentation.
Small Impoundments
Small impoundments primarily include farm ponds and small, off-river water supply impoundments. Most farm ponds in the southeastern United States were constructed in the last 50 years and represent nearly 0.5 percent of the land surface (Menzel and Cooper, 1992). About half of the surface area of small inland water bodies (those less than 16 ha or 39.5 acres) in Georgia is farm ponds (Menzel and Cooper, 1992). The damaging effects of farm ponds on native fishes are similar to those summarized for large impoundments except for negative impacts associated with hydrogeneration (U.S. National Research Council, 1992; Stanford and Ward, 1992). In most southern river systems the sheer number of farm ponds and their role in tributary fragmentation has not been sufficiently evaluated. In Settingdown Creek, an upper Piedmont tributary of the Etowah River, there are at least 13 farm ponds on order 1 and 2 tributaries (Figure 15). Although we have not estimated the total number of farm ponds in the Etowah River watershed, we surmise the density of farm ponds elsewhere in the system, except perhaps in the Blue Ridge province, is similar to Settingdown Creek.
Small off-river water supply impoundments are becoming an attractive source
of municipal water to growing communities and major metropolitan areas. Tentative
plans proposed to construct a series of reservoirs around the greater Atlanta
metropolitan area for future water supply. In the Etowah River watershed, plans
exist to impound Yellow Creek in eastern Cherokee and western Dawson counties
for water supply, and plans have been drafted for a similar impoundment on Sharp
Mountain Creek (Figure 16). The Yellow Creek impoundment will eliminate a healthy
population of the threatened Cherokee darter and further contribute to fragmentation
of the system. Water supply reservoirs on tributaries may also affect water
quality and flow in the main channel of the Etowah River, especially if water
is released to augment flow and availability at downstream municipal water intakes
during natural low-flow periods. Sharp Mountain Creek also harbors Cherokee
darters and the endangered amber darter, and it is one of the few remaining
large tributaries of the Etowah River system that is relatively undisturbed.
Sedimentation in eastern North America is often characterized as a form of nonpoint- source pollution (Karr and Schlosser, 1977). Herein we focus heavily on sedimentation because it is a widespread and serious problem in many fluvial systems. Unnatural sedimentation rates began with European colonization of North America. Sedimentation rates of Atlantic slope rivers are estimated to be four to five times greater than rates prior to European colonization (Meade, 1969). Intensive early sedimentation of the southeastern Piedmont was particularly associated with cotton farming and slavery and the fact that land was cheaper than labor; nutrient-exhausted fields were cheaper to abandon than to reclaim and exposed fields were simply left to erode (Trimble, 1974). Abandoned cultivated fields that were stripped of top soils typically became deeply gullied and in some cases it took as long as 75 to 100 years for erosion to be stabilized by natural revegetation (Trimble, 1974). Although overall current levels of sedimentation may be less than those in the first half of this century (Georgia Erosion and Sedimentation Control Panel, 1995; Pimentel et al., 1995), vastly accelerated sedimentation remains a ubiquitous threat in all southern upland river systems. The economic costs of soil erosion are staggering. Throughout the United States the annual costs of water-caused soil erosion is estimated to be about 7.4 billion dollars and annual costs for all causes of soil erosion are estimated to be 44 billion dollars (Pimentel et al., 1995). These estimates, however, do not consider the biological impacts of sedimentation and their intangible costs on humans. Herein we define sedimentation, examine the historical and present-day causes in the Etowah River watershed, and briefly review its effects on aquatic organisms.
Types of Sedimentation
Some biologists have synonymously referred to sedimentation and siltation (e.g., Burkhead and Jenkins, 1991; Bogan, 1993). Siltation is a form of sedimentation that refers to the deposition of silts (finely particulate soils) on stream or lake bottoms. Sedimentation encompasses a size range of solid particles including silt, sand, gravel, cobble, etc. Sediment transport along the bottom and suspended in the water column is a natural fluvial process of all rivers. The amount of sediment transported through a stream system is dependent on the quantity of sediment eroded into a stream and on the ability of a stream to carry washed-in sediments to a downstream outlet (Gordon et al., 1992). Increased sedimentation usually results from land-use practices that increase the amount of soil available to erosion. The total sediment load may be defined as the sum of bedload plus suspended bed-material load plus washload (Gordon et al., 1992). In southern upland streams bedload material consists of coarse substrates such as small rubble, gravel, and coarse sands that are moved along the bottom by rolling, sliding, or bouncing. Suspended bed-material load represents the smaller fractions of bedload that will remain in suspension for an appreciable time but that settle out when water velocity decreases. Washloads are the smallest sediments in particle size, such as fine sands, silts, and clays, and are readily suspended in low water velocity.
Washloads are considered supply-limited, i.e., dependent on bank or terrestrial sources of material, but are not limited by a stream’s capacity to carry them in suspension. Bed-material load is capacity-limited, i.e., dependent on channel morphology, discharge, gradient, and substrate composition and configuration (Gordon et al., 1992). Habitat-destructive portions of the total sediment load are the suspended bed-material loads that fall out of suspension during periods of stable or diminished flows. These sediments, when excessive at a level above a stream’s ability to flush them, can blanket the stream bottom and diminish the overall habitat complexity by filling interstitial spaces of the substrate. Fine silts, sands, and clays of the washload directly harm organisms by fouling mucous membranes of gills and egg surfaces. Species that are especially sensitive to high levels of sedimentation are those that evolved in upland streams where historic levels of sedimentation were usually low and water transparency was normally high.
Causes of Sedimentation
While geologists may consider sedimentation rates on temporal scales as large as one million to 100 million years (Meade et al., 1990), threats to southern Appalachian aquatic biodiversity due to increased sedimentation have only occurred in about the last 200 years. Prior to deforestation and early gold mining in the upper Coosa River system, the Etowah and Oostanaula rivers were probably as clear as the upper Conasauga River is today. Gold mining and associated erosion in the upper Etowah River caused the first major episode of sedimentation as early as 1830 (Leigh, 1994). Jordan (1922) described the main channel of the Etowah River as running muddy in 1876. A report by the Secretary of Agriculture to President Theodore Roosevelt described a correlation between poor logging practices and the clearing of mountain slopes to extensive sedimentation of southern Appalachian rivers (Wilson, 1902). We surmise that the Etowah River has been subjected to major sedimentation events since the 1830s, and has probably had greater-than-normal levels of sedimentation on a continual basis from the time the watershed was first settled by European immigrants.
Early deforestation within the Etowah watershed probably was not accompanied by efforts to abate sedimentation. By the early 20th century, upper Piedmont streams in Georgia, including the Etowah River, were exposed to extensive erosion from deforested uplands (Barrows et al., 1917). The solution to the resulting drainage problems was to ditch rivers and creeks to make them more efficient in transporting runoff (Barrows et al., 1917). The Soil Conservation Service (now the Natural Resources Conservation Service) channelized extensive areas of the Etowah River watershed. Bagby (1969) reviewed the deleterious consequences of channelization activities in Georgia including projects on 12 Etowah tributaries and the main river channel. The general effect of channelization on game fish populations was immortalized by the expression "you can’t get no fishes in SCS ditches" (Bagby, 1969).
The location of land-disturbing activities is important relative to inherent erosion vulnerability of soils. Meade et al. (1990) reported that deeply weathered Piedmont soils in Georgia produced ten times greater sediment yields than the poorly consolidated sedimentary soils of the Coastal Plain. The Upland subsection of the Piedmont represents the largest province in the Etowah watershed (Figure 3). Little River, one of the largest tributaries of the Etowah River (Figure 4) occurring entirely within the Upland subsection of the Piedmont, is probably the tributary most degraded by sedimentation in the watershed.
Current causes of sedimentation in the Etowah watershed are primarily construction, mining, and agricultural activities. Construction practices that leave soils (primarily red clays and sands in the Etowah River watershed) exposed to rainfall significantly increase levels of sedimentation (see Figure 17). Most recent construction is directly or indirectly associated with urbanization, industrial growth, and strip development along transportation routes. Urbanization of the watershed, especially around the greater Atlanta metropolitan area, increases rainfall runoff and greatly exacerbates sedimentation and pollution within the Etowah River watershed.
Most types of mining greatly accelerate sedimentation of rivers (Meade et al., 1990). Mining for bauxite, gold, iron, limestone, marble, and ocher has occurred in the Etowah River watershed (Butts and Gildersleeve, 1948; Park, 1953; Leigh, 1994). In the 1960s, one mining company in the vicinity of Cartersville discharged up to 550 tons per day of mineral-washing wastes into the Etowah River (Mackenthun, 1969). We have observed waste material from marble quarries in streams near Tate in Pickens County. Excessive sedimentation in these and other streams has occurred from mining and related construction activities, and has been intensified by denuded or thinned riparian cover (Figure 18).
Agricultural activities that contribute to excessive sedimentation include row-crop farming, livestock grazing, and silviculture. The amount of sediment entering streams is positively correlated with the percent of the drainage area in croplands (Meade et al., 1990).
Clearing of riparian vegetation is a ubiquitous problem throughout agricultural areas of the Southeast (Karr and Schlosser, 1977; Armour et al., 1991; Welsch, 1991). This greatly increases erosion (Figure 19) and is exacerbated in the mountains where steep slopes increase runoff rates and bank destabilization (Figure 20). Erosion from farms also contributes to water pollution and eutrophication, particularly where riparian zones have been cleared. Vegetated riparian buffer strips are efficient traps for sediments and nutrients (Lowrance et al., 1984, 1984a, 1984b, 1985; Lowrance, 1992). Most of the row-crop and livestock farming in the Etowah River watershed occurs in Piedmont and Valley and Ridge province subsections of Cherokee, Bartow, and Paulding counties. Euharlee, Raccoon, and Pumpkinvine creeks (Figure 4) are heavily degraded by these land uses.
Forestry activities contribute significantly to sedimentation in upland and
mountain streams through road construction and clearcutting (Schlosser, 1991;
Franklin, 1992). Historically, the U.S. Forest Service has been reluctant to
address sedimentation and hydrological changes of streams resulting from forestry
practices on federal lands (Hewlett, 1984). However, the Forest Service is demonstrating
a growing awareness of deleterious sedimentation problems relative to forestry
practices (Welsch, 1991; U.S. Department of Agriculture, 1994). Prospects for
increased sedimentation from forestry in the upper Etowah River watershed appear
to be significant. Due to timber harvest restrictions elsewhere in the United
States, the Southeast will likely face increased timber harvesting. However,
in the Etowah River watershed, approximately 85 percent of the land is privately
owned and timber quotas for federal lands may not have as great an impact as
in other southern Appalachian watersheds. Nonetheless, the Chattahoochee National
Forest encompasses some of the best remaining headwater streams of the Etowah
River, and increased clearcutting would exacerbate existing sedimentation problems.
Current timber harvesting in the Chattahoochee National Forest is causing a
large sediment load in the main channel of the Etowah River near the border
of the national forest at the Lumpkin County Route 72 bridge (authors’ pers.
obs.). Deep deposits of micaceous silts and sands are present at this site in
pools, and gravel, rubble, and small boulders are embedded in a matrix composed
of smaller sediments. The Georgia Forestry Commission recommends best management
practices (BMP) for timber harvesting on private lands. The use of BMPs is not
mandatory, and their effectiveness in reducing stream sedimentation from silvicuture
rests upon this voluntary compliance. Streamside management zones, which are
an integral part of these BMPs, vary in width from 25 feet on warmwater streams
to 100 feet on designated trout streams.
Destructive Consequences of Sedimentation
The destructive effects of excessive sedimentation on fluvially-adapted macroinvertebrates
and fishes have been well documented. Excessive sedimentation destroys habitats
by reducing habitat complexity and diversity. Substrates entombed by sediment
lose vital habitat niches for macroinvertebrates, thereby reducing community
and faunal complexity (Ellis, 1936; Cordone and Kelley, 1961; Chutter, 1969;
Nuttall and Bielby, 1973; Brusven and Prather, 1974; Fuller, 1974; Rosenberg
and Wiens, 1978; Quinn et al., 1992). Faunal decline caused by sedimentation
diminishes trophic web complexity and the efficiency of nutrient cycling within
the aquatic community. Fish biodiversity is similarly impoverished by the action
of sedimentation that reduces substrate heterogeneity. Because fish diversity
is positively correlated with habitat diversity (Gorman and Karr, 1978; Karr
and Dudley, 1981), increased sedimentation is particularly harmful to benthic
fishes (Berkman and Rabeni, 1987; see Figure 9). Moreover, catastrophic sedimentation
events can cause fish kills (Hesse and Newcomb, 1982). A particularly noxious
result of sedimentation is reduced reproductive success of benthic-spawning
fishes (Peters, 1967; Muncy et al., 1979). Egg survival in trouts is positively
correlated with intergravel permeability and increased particle size in the
redd, and increased mortality is largely associated with hypoxia from eggs and
larvae being smothered by fine silt (Chapman, 1988). Suffocation of eggs is
probably the primary cause of pre-larval mortality of benthic-spawning riverine
fishes.
Turbidity, caused by sediments suspended in the water column, reduces or eliminates light penetration thereby reducing primary photosynthetic productivity (Davies-Colley et al., 1992). Turbidity also affects sight-feeding fishes by diminishing their ability to detect prey. We speculate that many or some species exhibiting probable signs of sexual selection (such as the brilliant colors adorned by males of many species) may have reduced reproductive success in turbid water. Turbidity may also reduce the efficacy of a recently discovered, fascinating reproductive strategy in certain lampsiline mussels. Some species release superconglutinates — clusters of mussel larvae collectively mimicking the shape of a small fish or invertebrate attached to the end of a mucous strand — that sway and undulate in currents, acting as lures to potential host fishes (Haag et al., 1995). High turbidity would obviously reduce the ability of many fishes to detect such lures.
Prior to the U.S. Clean Water Act of 1972 (CWA) and subsequent amendments,
the Etowah River was moderately polluted in its middle and lower reaches, but
sections upstream from Allatoona Reservoir were considered relatively healthy
(Georgia Water Quality Control Board, 1970). Some of the most polluted sites
in the river were immediately below poultry rendering plants. For example, the
wet stream banks at a site in Blankets Creek below a poultry processing plant
in Canton were literally "scarlet from the protruding bodies of millions
of oligochaete worms" (Georgia Water Quality Control Board, 1970). Presently,
the Etowah River is significantly improved from the most severe cases of obvious
pollution that existed prior to the CWA. However, based on the tremendous decline
and extirpation of the aquatic biota, the present status of water quality is
probably not sufficient to maintain the existing fish and mussel biodiversity,
and water quality is clearly below standards necessary to restore the river.
We are unaware of any ongoing systematic monitoring of water quality in the Etowah River system other than that associated with municipal sewage discharges and water supply intakes. Primary point-source discharges in the river include sewage, industrial, and poultry processing effluents. Nonpoint-sources of pollution include excessive sedimentation from multiple sources, agricultural runoff (including pesticides and nutrients), mercury and other trace metals, potential landfill leaching (see Figure 16), and polycyclic aromatic hydrocarbons. The latter class of pollutants are primarily associated with urban and industrial environments (Neff, 1985). In this regard there is a direct relevance to human health concerns in the watershed. Because pesticides and trace metals can bind and accumulate in sediments (Leland and Kuwabara, 1985; Nimmo, 1985), we are concerned that the deleterious effects of the severe sedimentation problem in the Etowah River may be worsened by contaminants.
A serious future pollution threat to the Etowah River is the proposed interbasin
transfer of 42 million gallons per day (mgd) (1.74 ´ 108 lpd) of secondary-treated
sewage wastewater from the Chattahoochee River to the upper Etowah River in
Forsyth County, just above the mouth of Settingdown Creek (Figure 16). This
project was halted, at least temporarily, by the 1996 Georgia Legislature (Senate
Bill 500). This bill amends Georgia law to prohibit surface water interbasin
transfers of "sewage, industrial waste, treated wastewater, or other wastes"
unless the Director of the Georgia Department of Environmental Protection has
established criteria that allow for pollutants which cause receiving waters
or lakes below the point of discharge to fall below water quality standards.
In other words, a simple reduction of the waste-level standards would allow
for the transfer of treated sewage.
Using Forsyth County’s estimate of phosphorous (P) concentrations in the effluent, a 10 mgd (3.79 ´ 107 lpd) discharge would contribute an additional 4,145 kg (9,138 pounds) P per year to the Etowah River and Allatoona Reservoir, and 17,520 kg (38,624 pounds) P per year for a 42 mgd discharge. Measurements by one of us (BJF, October 1991) of P/PO4 at the proposed introduction site were 0.0055 parts per million (ppm), and recent measurements of P/PO4 from several Etowah River sites above the mouth of Settingdown Creek ranged from 0.0027 to 0.0122 ppm. A 42 mgd discharge would increase phosphorous during average flow conditions by 482 percent and by 1,682 percent during a 7Q10 flow (defined as the lowest flow on record measured during one week in a ten-year interval). The increases in nitrogen would be comparable. Clearly the proposed interbasin transfer would have a significant eutrophication effect on the Etowah River, and furthermore, the average temperature of the transferred wastewater would be 18.3° C (65° F), warmer than ambient water temperature in winter and cooler than ambient temperature in summer. Thus, the interbasin transfer would likely create additional stress and further contribute to habitat fragmentation of the Etowah River system by adding nutrient and thermal pollution.
The fact that the interbasin transfer could even be considered suggests that municipal and regional planners already view the Etowah River with little regard for its rich biological resources. In our opinion, a system-wide assessment of current water quality is critically needed, with special attention to the problem of contaminated sediments, and the establishment of a biological monitoring program for water quality standards.
The human population is projected to increase in the South by 31 percent by the year 2000 (Alig and Healy, 1987), and most of this growth will probably occur in major metropolitan areas. In few places does such projected growth approach the magnitude of the greater Atlanta area. Urban environments dramatically affect the physical characteristics of streams. Urbanization may increase sediment loads delivered to streams by as much as 100 percent (Meade et al., 1990). Because urban centers have large areas of impervious surfaces and infrastructure designed to efficiently transport water (e.g., gutters and storm drains), increased runoff rates enhance the transport of soils and other materials to stream channels (Hirsch et al., 1990). In Maryland streams, water quality impairment was first evidenced when watershed imperviousness reached 12 percent, and reduced water quality became severe when imperviousness reached 30 percent (Klein, 1979). Increased runoff rates in urban areas produce higher flood levels in short intervals, causing bank destabilization and erosion in outlet streams, and sometimes causing abrupt changes in channel morphology. Noonday Creek in Cobb County is an Etowah River tributary that drains an urban area (Figure 4). The substrates at sites we examined in Noonday Creek consisted entirely of clay and sand, 15 to 30 cm (5.9 to 11.8 inches) deep, covering the old gravel and rubble stream bottom. The substrate composition of streams in the Atlanta metropolitan area is also dominated by sand (Couch et al., 1995).
Streams draining urban landscapes typically have diminished, or significantly depleted, pollution-tolerant faunas (Duda et al., 1982; Weaver and Garman, 1994). Streams in the greater Atlanta metropolitan area have fewer native species, generally less than one-half, than intradrainage streams in predominately forested areas (Couch et al., 1995). The same Atlanta urban streams also have more nonindigenous species than similar streams in rural areas. Chemical by-products of urbanization, a plethora of chemicals ranging from used motor oil, paint products, and residential pesticides to PCBs leaked from transformers, have a variety of deleterious effects on aquatic organisms, including developmental abnormalities. Populations of the threatened Cherokee darter in Butler Creek, an Etowah River tributary in Cobb County, exhibited progressive interruption of the supratemporal canal (a sensory structure on the head) from the 1940s to present (Bauer et al., 1995). Interruption of the supratemporal canal is otherwise rare in subnose darters (Bauer et al., 1995), and may be a teratological phenomenon related to urban runoff.
Weaver and Garman (1994) suggested that urbanization represents a low-intensity disturbance of stream systems. While this is true relative to high-intensity disturbances such as large impoundments, catastrophic chemical spills, or point-source toxic discharges, the overall effects of urbanization can be equal to or more harmful to stream systems than high-intensity disturbances. The process of urbanization constitutes piecemeal habitat loss (see Faunal Fragmentation). The biological health of an urban stream depends on the type of urban environment the stream drains. Streams flowing through parks or semi-wooded residential areas with relatively intact riparian zones will retain more of their natural aquatic biota than streams flowing through concrete environments. The latter streams may be little more than culverts with faunas of hardy and pollution-tolerant organisms.
"Don’t it always seem to go
That you don’t know what you’ve got
Till it’s gone
They paved paradise
And put up a parking lot..."
— Joni Mitchell, Big Yellow Taxi
During Earth Week 1996, the nonprofit environmental organization American Rivers, Inc., released its annual list of the top ten most endangered river systems of the United States. Included on this list were the Etowah and the adjacent Chattahoochee rivers. These two systems, inextricably linked by proposed interbasin water exchange and mutual environmental problems associated with growth and extractive resource use, have the dubious distinction of being among the most ecologically threatened rivers in the Southeast. This treatise is timely considering the inauspicious recognition of the Etowah River as one of the nation’s most endangered river ecosystems. Herein, we provide an overview and summary of basic tenets of restoration ecology as applied to river systems and adjacent landscapes, and conclude with a variety of topics to be considered by all citizens and organizations with vested interests in the Etowah River watershed and other southern Appalachian rivers.
Restoration ecology is an important and fast growing subdiscipline of conservation biology. Management of natural resources has long included aspects of habitat recovery, but only relatively recently has there been heightened attention to proactive, comprehensive restoration of habitats, communities, and ecosystems. The following examples illustrate some of the reasons for a greatly accelerated focus on riverine restoration ecology: 1. Even though the U.S. Clean Water Act was established over two decades ago, today nearly half of the nation’s fresh waters fail to meet minimum water quality standards based on biological criteria; 2. Less than two percent of all lotic river miles in the United States currently receive federal protection under the 1968 U.S. National Wild and Scenic Rivers Act; 3. Principal objectives of the 1973 U.S. Endangered Species Act are to list, protect, and recover imperiled plants and animals, yet listing and recovery of most species and populations has lagged far behind discovery and recognition of jeopardized taxa, such that aquatic species, in particular, are now disappearing rapidly and ubiquitously (Doppelt et al., 1993; Warren and Burr, 1994; Mann and Plummer, 1995; Walsh et al., 1995).
As a consequence of the general failure of existing single-species management programs, most scientists now espouse greater conservation efforts toward multiple scales of biological complexity. Much of this shift in emphasis is a consequence of increasingly diminished global biodiversity and a greater realization that effective conservation programs must include protection of relatively healthy habitats and recovery of perturbed habitats. Emerging as a vital activity of more encompassing resource management, ecological restoration is a rational approach for recovering, reestablishing, and protecting native biotas (U.S. National Research Council, 1992; Doppelt et al., 1993; MacMahon and Jordan, 1994; Noss and Cooperrider, 1994).
Increasingly, restoration programs target entire ecosystems. Our concept of an ecosystem generally follows that of Whittaker (1975) and Odum (1993), and is broadly defined as an aggregate of communities and environments treated together as a functioning system of complementary relationships that transfer and circulate energy and matter. We stress that this definition includes all dynamic spatial and temporal processes that affect natural, interconnected terrestrial and aquatic assemblages. Additional, concise definitions related to riverine watersheds and riparian areas provided by Doppelt et al. (1993) are used similarly here, to encourage people to think about "riverine systems as complex, dynamic ecological and biological systems on a landscape scale." These authors use the terms "watershed ecosystem" and "riverine-riparian ecosystem" in a hierarchical sense; these definitions and other relevant terms are summarized in Table 5.
During its early growth, the field of restoration ecology has undergone extensive theoretical development. The objectives, scope, limitations, and feasibility of ecosystem restoration have been broadly examined. Considering the obstacles and complexity of restoration, some ecologists argue that successful restoration of seriously degraded habitats on a large scale is unlikely or impossible. Notwithstanding, most ecologists feel that restoration must be a common goal of future conservation activities. In southern Appalachia there is an acute need for riverine restoration to protect the remaining aquatic biodiversity of the region. For practical purposes, we adhere loosely to many proffered definitions of "restoration," "rehabilitation," "reclamation," "recovery," and related terms (U.S. National Research Council, 1992; MacMahon and Jordan, 1994). The term "restoration" itself is generally used to mean the return of an ecosystem to a close approximation of its condition prior to disturbance (U.S. National Research Council, 1992). Realistically, we acknowledge that few if any southern rivers may ever return to historic conditions or a reasonable semblance of that which existed prior to human settlement. Thus, we employ the term "restoration" as the sum of all processes and activities that serve to redirect an ecosystem on a trajectory toward a pre-disturbance state (MacMahon and Jordan, 1994). In practical terms, we believe the preferred endpoint should be to restore conditions that maximize biological evolutionary processes through space and time. The term "recovery" is also used here in a broad sense, to mean not only the return of natural conditions through passive processes (MacMahon and Jordan, 1994), but also through active human intervention such as programs to abate excessive sedimentation or to reintroduce extirpated species. We refer the reader to the following synoptic works, and references in each, for additional general information on ecological restoration and watershed management: Gore, 1985; Jordan et al., 1987; Naiman, 1992; U.S. National Research Council, 1992; Doppelt et al., 1993; Hesse et al., 1993; Cairns, 1994; MacMahon and Jordan, 1994; and Noss and Cooperrider, 1994.
The Etowah River watershed presents formidable challenges for ecological restoration due to a burgeoning human population and widespread negative environmental changes within the watershed and in northwestern Georgia. Below we present a general prospectus for planning, prioritizing, and initiating restoration activities within the Etowah River watershed. Our intent is to provide a general framework on which to eventually build a more detailed, consensus-based strategy for restoration and ecosystem management of aquatic resources in the Etowah River system. This framework should have direct application to restoration of other riverine systems throughout the southern Appalachians.
The National Research Council developed a checklist of important questions, goals, and criteria to be addressed prior to, during, and following any restoration program (U.S. National Research Council, 1992, Table 3.1; MacMahon and Jordan, 1994, Table 14.1). These central questions concern various aspects of each project’s mission, planning, design, feasibility, scope, cost, post-restoration analysis, and long-term ecological and socioeconomic benefits. However, restoration needs of the Etowah River are so extensive that many of the specific questions outlined by the U.S. National Research Council (1992) at present cannot be adequately addressed to determine the suitability and potential success of individual restoration projects within the watershed. Moreover, environmental protection of the Etowah River watershed has long been neglected and there have been no previous comprehensive efforts to identify and initiate ecosystem restoration programs. We feel that a top priority exists for coordination of local and regional assessments, planning, and management of growth and environmental protection within the Etowah River system. Better organization and communication than currently exists among all relevant parties is a necessary first step to initiating restoration projects within the ecosystem, since restoration efforts cannot be expected to succeed without enhanced coordination of resource management (Ford et al., 1990; Doppelt et al., 1993). Ultimately, successful recovery will not occur unless private citizens, the business sector, and government agencies unite with a mutual goal of restoring ecological integrity of the Etowah River watershed.
The restoration and watershed management goals that we envision for the Etowah River ecosystem are straightforward: to reverse as much as possible all conditions contributing to the decline of natural, physical, and biological resources and processes; to protect minimally disturbed areas, and; to stem future losses of biodiversity and diminution of ecosystem health. Given the magnitude of ecological degradation and the extent of urban and rural growth in the area, it is unrealistic from a practical standpoint to assume that comprehensive, system-wide ecological restoration throughout the Etowah River watershed is possible in the immediate foreseeable future. However, rather than adopting a minimalist approach to restoration, we believe the above stated objectives impart ultimate goals to strive for, and discourage compromising attitudes that certain portions of the drainage are unrecoverable, can be sacrificed, or excluded from restoration activities.
The greatest limitations facing restoration in the Etowah River watershed, as in most other systems, are fiscal constraints, the coordination of public and private interests, and the balancing of socioeconomic concerns. Meffe and Carroll (1994, page 492) define sustainable development as "human activities conducted in a manner that respects the intrinsic value of the natural world, the role of the natural world in human well-being, and the need for humans to live on the income from nature’s capital rather than the capital itself." This is analogous to living on the dividends of one’s investments rather than the principal itself; the term "sustainable development" is often grossly misunderstood and fallaciously misused by politicians and the corporate sector. The main threat to the biological and physical integrity of the Etowah River system is unsustainable growth in northwest Georgia. Ecological restoration efforts in the watershed will be successful over time only if there is acceptance of mutual responsibility and commitment by citizens, the business community, and government agencies to plan and better manage economic development, and, in our opinion, to seriously address consumptive resource use in the region.
Prudent analysis and planning of aquatic ecosystem restoration is fundamental to the success of any program. For the Etowah River watershed, the need for a unified, aggressive restoration and ecosystem management plan has never been greater than now. Various authors have discussed the value and nature of integrative planning processes and methods that involve policy analysts, decision makers, resource managers, and scientists (U.S. National Research Council, 1992; Doppelt et al., 1993). Most such programs would encompass all relevant economic, social, and environmental considerations early in the assessment process, while retaining significant flexibility (Holling, 1978). Recently, Doppelt et al. (1993) proposed a new approach to riverine restoration — the Rapid Biotic and Ecosystem Response — a concept founded on principles linking watershed dynamics, ecosystem function, and conservation biology. Their approach involves three integrated components. Initially, there is comprehensive identification and protection of remaining, relatively healthy tributaries, biotic refuges, riparian areas, floodplains, and biological "hot spots" throughout the entire ecosystem. This emphasizes protection and reduces the need for post-disturbance control or repair, thereby improving effectiveness and cost-efficiency. Second, restoration efforts are devoted to improved management of intervening areas between those that are protected, with a goal to eventually link healthy areas. A third concurrent step in the Rapid Biotic and Ecosystem Response is to actively involve local communities and citizens in implementing all the steps for planning and supporting environmentally sustainable economic development. This approach is urgently needed to begin ecological restoration of the Etowah River watershed.
Considerable debate exists concerning minimum reserve size for protected areas, extent and nature of habitat corridors, and appropriate scales for ecosystem management and restoration. Some of the most successful or widely publicized restoration projects have been relatively small in scale, on the order of a few hectares, and most riverine restoration projects are limited to rehabilitation of selected river sections to a predetermined state of structure and function (Gore and Shields, 1995). Moreover, many ecosystems are so severely degraded or overexploited that socioeconomic and other factors limit the practicality of comprehensive, large-scale restoration projects. Nevertheless, riverine restoration programs should ultimately be done on a watershed- or ecosystem-wide basis (e.g., Noss and Cooperrider, 1994). At the very least, restoration planning should encompass the entire ecosystem, even if initial restoration activities are limited to subsections. In fluvial ecosystems this is especially important, because environmental impacts in one part of the catchment may strongly affect other areas of the watershed. For example, mitigation of major sedimentation sources in upper portions of a watershed will positively affect downstream reaches.
Potential success of ecological restoration projects is directly related to spatial scale according to the following criteria: 1) The project area must be sufficiently large to ameliorate deleterious effects that boundary conditions may impose on interior aquatic functions. 2) Project managers must have control or influence over zones where major causes of ecological disturbance exist. 3) The area must be large enough to allow for monitoring and follow-up assessment of success. 4) A project must be affordable in size (U.S. National Research Council, 1992).
In the Etowah River system many environment-degrading land practices are widespread and restoration might best be approached on a watershed-wide basis, such as by establishing programs for reducing loss of riparian vegetation, nonpoint-source pollution, and sedimentation. Other problems are very site-specific and restoration could be enhanced by mitigation on a relatively small scale, such as upgrading sewage treatment facilities. A salient aspect of the planning process is to evaluate and rank river reaches and tributaries relative to their ecological status and restoration needs.
A fundamental premise of ecological restoration is that background information exists regarding predisturbance conditions of the ecosystem. While the precise historical structure of an ecosystem is seldom if ever known, some estimate can be made by extrapolating from conditions that persist or by comparison with similar systems. Unfortunately, ecologists rarely have sufficient baseline data to accurately reconstruct historical conditions or to evaluate community structure and dynamics on the basis of comparable systems. Ideally, planning a restoration project includes survey, inventory, and compilation of preliminary physical and biological data to assess progress and success. In practice, however, cost limitations and the degree of environmental change may preclude adequate pre-restoration analysis of existing or historic conditions. However, obvious ecologically-deleterious conditions do not require knowledge of predisturbance circumstances in order to begin fundamental steps of restoration, assuming disturbance causes are adequately understood and restoration goals have been established.
Crucial information about the Etowah River watershed is currently lacking. Basic biological and geophysical research is needed to determine present ecosystem structure and dynamics. Also needed are assessments of current land-use coverage and general environmental conditions, as well as a comprehensive review of all existing municipal and regional growth and development policies and plans. Several important initiatives are required for greater organization of resource management and restoration in the Etowah River watershed (Table 6). These initiatives mainly fall under general categories of political and socioeconomic review, ecosystem evaluation, restoration planning, and public education; these goals are common in any restoration program and need to be seriously addressed for the Etowah River ecosystem. To coordinate environmental reform across the watershed, we propose that a well-balanced organization be established to review, plan, and implement improved ecosystem management. An "Etowah Watershed Alliance," or similarly-named organization should be composed of individuals and groups representing diverse economic, environmental, and political interests, with a common goal of establishing healthy ecosystem management while striving for true sustainable development. Bolling (1994) provides important considerations in building a successful, interjurisdictional program for watershed protection and management.
Table 5. Definitions of physical and biological terms pertinent to riverine ecosystem restoration and management, slightly modified from Doppelt et al. (1993). |
|
Term |
Definition |
biological diversity (biodiversity) |
The variety of the world's biological elements and processes, represented and integrated over organizational levels from genes to landscapes. |
biological hot spots |
Intact riverine habitat patches that provide critical functions for biodiversity or the stream; ranging from microhabitats (such as individual pools or riffles) to larger sections of complex, healthy habitats. |
biotic refuges (refugia) |
Physical areas with healthy and relatively undisturbed habitats and processes that serve as refuges for biodiversity. |
ecosystem simplification |
The cumulative result of impacts causing large reductions in the life-supporting complexity and diversity of ecosystems. |
riparian area |
The transition zone between the flowing water and terrestrial ecosystems. |
riverine-riparian biodiversity |
All native aquatic and riparian organisms dependent on the riverine-riparian ecosystem. |
riverine-riparian ecosystem |
All the processes and elements that interact in the flowing water and riparian areas of the riverine system (often corresponding to the 100-year floodplain). |
riverine system |
An entire river network, including all tributaries, sloughs, side channels, and intermittent streams. The term riverine is used more restrictively than aquatic to mean only a natural flowing freshwater system. |
watershed (catchment basin) |
The entire physical area or basin drained by a stream or riverine system, separated from other watersheds by ridgetop boundaries. |
watershed ecosystem |
All of the elements and processes that interact within the watershed. |
Whereas cooperative alliances, some planning, and initial restoration measures can be immediately implemented, critical research is needed for organized, cost-effective restoration throughout the Etowah River watershed. Research needs span multiple and complex areas, including species biology, community and system ecology, hydrology, fluvial geomorphology, and human demographic and socioeconomic issues. An exhaustive analysis of research priorities for the Etowah River watershed is beyond the scope and intent of this work, but we summarize the primary research deficiencies that need to be addressed in Table 7.
Basic research required to determine the current ecological health of the watershed are analyses of land-use coverage and determinations of biotic diversity, location, and abundance. Several valuable analytical tools are applicable for this assessment, including Aquatic Gap Analysis, Population Viability Analysis, and the Index of Biotic Integrity (Table 7). Surveys of aquatic macroinvertebrates are especially needed for incorporation in Aquatic Gap Analysis. There is a strong need for determining the impacts of habitat fragmentation on the genetic structure of metapopulations, and for evaluating habitat discontinuity and barriers to potential dispersal and recolonization capabilities of aquatic organisms. This is especially important for determining species at greatest risk and for identifying critical linkage areas to target for restoration. Basic autecological research to determine habitat requirements and life histories of selected species representing different ecological guilds is required for planning comprehensive restoration projects and in evaluating the recovery progress. Typical species-oriented restoration approaches focus on habitat rehabilitation for a few high-profile species. This type of restoration effort is often endorsed because ecological data are lacking for many species, or management is aimed toward game or protected species. Such efforts to optimize habitats for a few valued species may cause suboptimal conditions for other species or impair riverine ecology, and may therefore be inconsistent with goals of ecosystem management in maintaining or recovering biological integrity (Keenlyne, 1993; Sparks, 1995).
Important toxicological research should focus on elucidating the effects of sediments and both organic and inorganic contaminants on survivorship, reproductive success, and other ecological traits of aquatic species. These data are especially needed for the early life-history stages of riverine organisms in the Etowah River watershed. Experimental data are essential, since qualitative conclusions about the effects of sediments are often unsubstantiated by empirical data and do not improve scientific understanding of the biological basis for negative ecological impacts of sediments (Berkman and Rabeni, 1987). Toxicological studies should emphasize the synergistic effects of chemical pollutants and sediments that are often bound together during transport or deposition (Leland and Kuwabara, 1985; Nimmo, 1985).
There is an immediate need for determining physical structure and hydrological function of the Etowah River watershed to better understand ecological impacts. A preliminary analysis should be done to assess historical changes that have occurred and the current status and conditions of both aquatic and terrestrial resources. Pervasive riparian loss and sedimentation throughout the drainage indicate that a comprehensive geomorphological study is in order. Especially urgent is an analysis of sediment, contaminant, and nutrient transport throughout the system, so that sources and sinks can be identified and targeted for mitigation. Sediment sampling and transport modeling are increasingly essential to riverine restoration and watershed management (Gordon et al., 1992), and are imperative for the Etowah River watershed because of the highly erodible soils of the Piedmont province, riparian deforestation, and overall deteriorating water quality of Georgia’s rivers (Meade et al., 1990; Georgia Erosion and Sedimentation Panel, 1995). We recommend that the Etowah River watershed be seriously considered for inclusion in the National Water-Quality Assessment (NAWQA) program of the U.S. Geological Survey. The NAWQA program evaluates physical and biological conditions of surface and groundwater resources, thereby providing critical baseline data necessary for resource assessment, restoration planning, and long-range monitoring of water quality. The recent addition of the Etowah River to the NAWQA program is especially timely due to the rapid rate of ecosystem decline in the Etowah River system, and imminent prospects for greatly accelerated demands on water resources in the greater Atlanta area.
The most challenging research needs for the Etowah River watershed involve socioeconomic issues. Because of highly fragmented and often conflicting government and private interests, there is currently no comprehensive ecosystem management of aquatic and terrestrial resources. In fact, we are unaware of any metropolitan areas in or adjacent to the watershed that consider principals of resource sustainability during economic planning. The first step in initiating ecosystem restoration of the Etowah River watershed is to review existing environmental policies at all levels, and to collectively draw together all public and private partners with vested economic and natural resource interests. Accordingly, we have listed in Appendix 1 many of the relevant organizations and government agencies with significant environmental responsibilities for the Etowah River watershed and general interests in environmental conservation. One function of a watershed coalition (see Restoration Framework above) would be to serve as a liaison between these diverse groups and to focus them on a unified, comprehensive approach to ecosystem management and restoration.
Within the Etowah River watershed, as elsewhere throughout southern Appalachia, the following criteria are among the most important factors in considering specific riverine areas to target for restoration: 1. existing or historical biotic diversity and endemism; 2. nature and extent of ecological perturbations and degradation; 3. biological, physical, and socioeconomic value of restoring a specific area; 4. potential for success in restoring an area; and 5. cost effectiveness relative to improving overall health of the ecosystem.
The complex land-use pattern that surrounds the Etowah River provides a mosaic from which to identify and establish priority areas suitable for ecosystem protection and ecological restoration. General categorization of subregions of the Etowah River watershed based on environmental perturbation and existing fish diversity provides a general basis for targeting restoration areas (Figure 21). Relatively high-quality areas have the least ecosystem damage and retain the greatest degree of faunal diversity and endemism, or least extirpation of native taxa. These are primarily tributary headwaters in the upper portion of the watershed that have large, intact riparian zones forested with native hardwood species and where disturbances are generally least intensive. Included in this category are headwaters and adjacent lands of the Etowah River system, encompassing all or significant portions of Sharp Mountain Creek, Long Swamp Creek, Amicalola Creek, and Shoal Creek. Faunal diversity remains fairly high and endemic fishes (three species of snubnose darters and the Etowah darter) are found in these regions. Also placed in this category are two areas in the lower portion of the watershed, Spring Creek and Connesena Creek, which have undergone moderate habitat degradation but harbor species (rainbow shiner and blacknose dace) that are restricted or extirpated elsewhere in the Etowah River system and are otherwise rare or uncommon throughout the upper Coosa River system.
Consideration of the above regions as areas of relatively low restoration priority is not intended to diminish their ecological importance. Conversely, the least-perturbed areas of the Etowah River watershed are among those with the highest intrinsic biological, physical, and aesthetic value. For this reason, these are regions that should be protected through strong, proactive conservation measures. Protection of the relatively undisturbed and healthier headwaters, tributaries, riparian zones, and biological hot spots is essential to the future success of other restoration activities and is the first strategy in the Rapid Biotic and Ecosystem Response approach of Doppelt et al. (1993). Long-range monitoring of these areas should be done to ensure that negative environmental impacts are minimal, and the areas should be targeted for restoration efforts if present conditions deteriorate. Significant areas for long-term protection are in the headwaters of the Etowah River system within the Chattahoochee National Forest. The U.S. Forest Service should manage federal lands under its jurisdiction within the region by implementing Best Management Practices that beneficially contribute to overall ecosystem health (see Remediation below).
Of somewhat higher priority for riverine restoration within the Etowah River
watershed are regions with moderate environmental degradation and faunal decline.
These areas are in the upper-middle portion of the drainage, from Stamp Creek
in northeastern Bartow County to the main channel and southern tributaries upstream
of Allatoona Reservoir in the northern half of Cherokee County and southern
portions of Pickens, Dawson, and Lumpkin counties. Also included in this category
are headwaters of various lower Etowah River tributaries in Paulding, Polk,
Floyd, and Bartow counties (Figure 21). These are largely rural, agricultural
areas, although significant portions of Cherokee, Pickens, Dawson, and Lumpkin
counties remain moderately forested with mixed hardwoods and conifers. The extreme
lower Etowah River and Spring Creek have been detrimentally impacted by industrial
development and urbanization near the city of Rome. In Cherokee County, there
has been recent and rapid urban sprawl extending northward along a corridor
centered around Interstate 575. Additionally, the planned northern perimeter
loop of the interstate highway system will bring further development and associated
negative impacts to the northcentral part of the watershed.
Restoration planning in the above moderately-impacted areas minimally should include three primary facets. First, immediate efforts should be made to evaluate present land-use coverage and to identify and secure protection for existing stream reaches with maximum physical and biological integrity and ecosystem health. Second, degraded stream sections that could potentially link the best-quality habitats should be identified, ranked, and targeted for specific cost-effective rehabilitation projects. Third, methods should be developed and implemented to abate, mitigate, and reverse the most pervasive causes of environmental degradation, especially reducing the loss of riparian cover and improving control of sediments, nutrients, and pollutants emanating from farms, landfills, golf courses, and urban development. There should be careful scrutiny of existing and future proposed projects that are likely to have negative environmental impacts in these areas, with critical attention devoted to exploring economically viable alternatives. We suggest that serious consideration be given to a temporary moratorium on proposed water-supply impoundments in areas of the basin pending a comprehensive review of projected growth and water usage needs throughout the drainage. Research should also be initiatied on practical conservation measures and alternatives to construction of new reservoirs, and evaluation of long-range impacts of each additional proposed impoundment.
Restoration efforts within the moderately-impacted areas are critical for ecosystem recovery of the entire watershed, because continued ecological decline in tributary headwaters of these sections will further exacerbate detrimental impacts downstream. Further, these stream segments and riparian zones are critical linkage areas and their restoration is essential for health of the entire watershed. Moreover, restoration projects in these areas could be the most cost-effective, in terms of potential for rehabilitation success and overall positive impacts to the watershed relative to per capita expenditures.
Ecological conditions are most severely degraded in a large, central portion of the Etowah River watershed (Fig. 21). Included in this area are streams that have undergone faunal impoverishment and ecosystem simplification through habitat loss and cumulative effects listed under Threats to the System, primarily resulting from Allatoona Reservoir, urbanization, deforestation and destruction of riparian cover, industrial and agricultural pollution, widespread sedimentation, and eutrophication. Considerable growth in recent years between Marietta (Cobb County) and Cartersville (Bartow County) has contributed significantly to environmental decline in the central portion of the drainage, coupled with years of heavy agriculture. Etowah River tributaries in Fulton and Forsyth counties have been so severely modified by harmful agricultural effects, urbanization, and small impoundments, that sedimentation and other factors have reduced faunal diversity to a disturbance-tolerant guild, much like nearby streams in urban reaches of the upper Chattahoochee River system (Couch et al., 1995). Major Etowah River tributaries that fall in the high-disturbance category include Euharlee Creek, Petit Creek, Little River, Noonday Creek, and lower Pumpkinvine Creek. Additionally, direct tributaries flowing into Allatoona Reservoir, such as Stamp Creek and Allatoona Creek, have suffered extensive degradation and have depauperate faunas that are isolated from other tributaries of the drainage. Finally, Allatoona Reservoir has obliterated large sections of fluvial habitats and has a significant effect on the main channel fauna far downstream of the dam, as discussed under Threats to the System.
Restoration of heavily-disturbed areas presents some of the most challenging problems to ecosystem management in the Etowah River watershed. Many of the major degraded riverine segments are critical to ecosystem function and could provide major linkages between healthier regions of the watershed. These highly altered sectors are bound to primary economic development in the drainage, and their rehabilitation involves the greatest social, political, and financial obstacles. As the most seriously impacted riverine segments, these areas will require the greatest amount of work and time to successfully restore. Moreover, restoring these streams will not only be expensive, but some restored habitats, especially tributaries flowing into Allatoona Reservoir, will not be available to colonizing fishes from other tributaries or the main channel. Likewise, fish populations restored by introductions could not serve as sources to other tributaries or the main channel without human intervention. Thus, restoration projects in the most perturbed areas may not be the most economical or practical, and socioeconomic costs must be thoroughly evaluated relative to potential success for ecological restoration and the overall impact of improving ecosystem function.
Table 6. List of major steps needed to develop a regional ecosystem restoration and management strategy for the Etowah River watershed. |
|
a. |
Identify all appropriate individuals, institutions, industries, and agencies within the private and public sectors to participate in ecosystem management and restoration within the watershed (see Appendix 1). |
b. |
Adopt and implement a comprehensive public education program. |
c. |
Evaluate present status of aquatic and terrestrial biological and physical resources, including faunal surveys, basic ecological research, indices of biological integrity, and risk assessment. |
d. |
Determine complete hydrologic discharge, removal, wastewater treatment, and water quality throughout the watershed, including a review of all existing residential and industrial wastewater and point-source permits. |
e. |
Establish long-term water quality monitoring stations on all major tributaries and along the entire main channel of the Etowah River. In accordance with the National Water-Quality Assessment (NAWQA) program of the U.S. Geological Survey, critical sites should be established for modeling sediment transport and water quality. |
f. |
Compile and analyze existing land-use data and proposed agricultural and municipal projects with potential adverse environmental impacts. A centralized data base would facilitate restoration planning and comprehensive resource management strategies. |
g. |
Evaluate, adopt, and implement regional growth management plans for the multi-county metropolitan and rural corridors within and surrounding the Etowah River watershed. |
h. |
Develop comprehensive assessment, planning, and monitoring programs for management of sustainable natural resources within the region. Coordinate local municipal, county, and state government agencies, industries, real estate speculators, and landowners in developing a watershed-based environmental management plan. |
i. |
Review and revise all local and regional regulatory policies governing environmental protection and natural resource management. |
j. |
Identify, prioritize, target, and modify environmentally detrimental land-use practices. Revise urban zoning procedures. |
k. |
Evaluate, rank, and establish individual geographic areas for pilot restoration projects. |
l. |
Analyze and secure cooperative funding from all potential sources; establish a financial review panel for identifying and soliciting suitable funding sources. |
m. |
Recruit and engage all available industries, agencies, conservation organizations, and private citizens in conducting and monitoring individual restoration projects. |
Based on the above review of restoration concepts and issues, we suggest the following measures for consideration as a starting point for comprehensive and organized restoration of the Etowah River watershed. We feel there is an urgency for certain immediate, remedial restoration activities to prevent continued ecological degradation and possible irreversible loss of biological diversity and ecosystem function. The following list of possible remedial measures for the Etowah River watershed is far from being exhaustive, but addresses some of the basic and most important restoration issues that we feel pertain to the Etowah River watershed. This outline is not intended to be a mandate or to establish priorities. Rather, it is intended to provide a focal point and catalyst for open debate, critical evaluation, and, hopefully, earnest public action devoted to ecosystem restoration efforts within the basin. All of the measures detailed below should be construed as viable options suitable for serious consideration and constructive scrutiny in an open forum of all public and private parties with vested interests in land-use and environmental issues of the Etowah River ecosystem.
I. Organization of an ecosystem-protective coalition (Etowah Watershed Alliance).
A. Assemble relevant private, business, and government interests (Appendix 1).
B. Plan and conduct annual public conferences on socioeconomic and natural
resource issues pertaining to the Etowah River watershed.C. Formulate short- and long-range plans for sustainable development within the
watershed.II. Sedimentation abatement.
A. Adopt and apply research, monitoring, and mitigation recommendations of the Georgia Erosion and Sedimentation Control Panel (1995).
B. Involve existing programs of the U.S. Fish and Wildlife Service (Partners in Wildlife), the Natural Resources Conservation Service, the National Fish and Wildlife Foundation, The Nature Conservancy, and various public and private endowments to assist landowners in protecting and recovering riparian zones.
C. Provide incentives and education to farmers for mitigating and abating sedimentation and topsoil erosion.
D. Establish standards for vegetated riparian buffer strips along streams in each physiographic province.
E. Encourage and provide incentives for private and business landowners to replant native vegetation in currently denuded riparian areas.
F. Promote effective sediment-control measures during all types of building construction, roadway development, and other forms of habitat modification.
G. Adopt and implement best management practices (e.g., Bisson et al., 1992; U.S. Department of Agriculture, 1994) on federal lands in the Chattahoochee National Forest with emphasis on protection of aquatic habitats.
1. Plan, develop, and adapt management practices geared specifically toward ecological protection of southern Appalachian riverine resources.
2. Investigate economically viable alternatives to cultivation of conifer monocultures that promote silviculture methods utilizing native hardwood species.
3. Evaluate all clearcutting and road-building methods and implement harvest techniques that minimize sediment production.
4. Promote fisheries management programs that favor diversity, abundance, and enjoyment of native aquatic communities without comprimising recreational, commercial, or tourism interests.
H. Provide incentives to mitigate industrial and mining sources of sedimentation.
III. Minimizing impoundment and runoff effects.
A. Investigate all potential ecological, socioeconomic, and associated costs and benefits that might pertain to construction of a re-regulation dam below Allatoona Reservoir to mitigate flow pulses in the main river channel.
B. Evaluate potential feasibility of installing an air-injection system, or a suitable alternative, in Allatoona Dam to alleviate low dissolved oxygen and high hydrogen sulfide concentrations in the reservoir tailrace.
C. Conduct exhaustive reviews of existing and future water demands and reevaluate the necessity for new water-supply impoundments.
D. Educate, encourage, and provide incentives to private citizens and businesses to adopt beneficial water and energy conservation measures.
E. Investigate suitable alternatives to locating new urban developments, roadway construction, and other impervious surfaces near or adjacent to the main river channel and tributaries.
F. Create incentives for attracting low water-consumptive industries to the area.
IV. Pollution mitigation.
A. Upgrade all municipal sewage treatment facilities for tertiary treatment.
B. Review existing National Pollution Discharge Elimination System permits, and monitor minimum water quality standards for all licensed point-source discharges throughout the watershed.
C. Provide incentives to poultry farmers and processing facilities to alleviate negative impacts of waste disposal.
D. Discourage placement of any new landfills, golf courses, or sources of industrial pollutants adjacent to or near the main river channel or tributaries.
E. Explore alternatives to interbasin transfers of wastewater.
V. Ecosystem protection.
A. Review potential for seeking designation of Amicalola Creek as a National Wild and Scenic River.
B. Pursue opportunities for acquisition and protection of additional public lands.
C. Educate, encourage, assist, and empower private landowners, citizen groups, and conservation organizations in protecting local natural resources.
Table 7. Summary list of critical research needs for the Etowah River watershed. |
ECOLOGICAL |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GEOPHYSICAL |
|
|
|
|
|
|
|
|
|
SOCIOECONOMIC |
|
|
|
|
|
|
|
|
Pervasive environmental deterioration of the Etowah River watershed is partly attributable to inadequate education about the value and significance of ecosystem protection and the benefits of sustainable development. Like many places elsewhere, a lack of awareness about the plight and importance of the Etowah River watershed contributes to continual disregard for the long-range effects of unsustainable exploitation of natural resources within the ecosystem. Ecological restoration cannot be expected to succeed without greater public education and galvanized support for a conservation agenda to protect the Etowah River watershed.
Improved education about the current status and future prospects of the Etowah River watershed encompasses two priorities: education of the general public, and education of policy makers and natural resource managers. Public education must include programs that purvey the current status and role of the watershed to overall ecosystem function, and how ecosystem health relates to long-term human welfare. Central to general education is the notion that public empowerment is essential to solving environmental problems. As stewards entrusted by the public, natural resource agencies and policy makers must be aware of all environmental issues affecting the watershed and must take strong responsibility and leadership in holistic ecosystem management.
Protection of the Etowah River watershed, like all other natural ecosystems, may require a fundamental change in how local citizens view their role in the global environment. Humans are as equally susceptible as many other organisms to disease, famine, natural disasters, and, especially, overpopulation. As sentient beings, there is a prevalent myopia that we are able to solve all technological, biological, and other challenges, and that materialistic gain and human welfare supersedes the environmental consequences of unsustained exploitation of natural resources. Yet, many environmental education programs fail to stress that human existence is ultimately and inextricably linked to a well-balanced global biosphere, comprised of functional and healthy ecosystems. As Rolston (1991) eloquently stated, "There is something overspecialized about an ethic, held by the dominant class of Homo sapiens, that regards the welfare of only one of several million species as an object and beneficiary of duty...about living in a reference frame in which one species takes itself as absolute and values everything else relative to its utility. If true to its specific epithet, which means wise, ought not Homo sapiens value this host of life as something that lays on us a claim to care for life in its own right?" If precious natural resources of the Etowah River system are to be saved, people in and around the watershed must cogently acknowledge that ecological protection and environmental sustainability are intrinsically valuable and essential for a healthy quality of life for all organisms within the ecosystem.
We are grateful to the Georgia Department of Natural Resources, especially G. S. Beisser and R. M. Gennings, for granting scientific collecting permits, field assistance, and technical support. The U.S. Fish and Wildlife Service provided most of the funding and technical assistance for surveys of the Cherokee and Etowah darters. Additional funding was also provided by the U.S. Forest Service. We especially thank the following individuals for aid in field work: M. M. Bentzien, R. T. Bryant, J. H. Chick, R. S. Cowles, A. Daniels, G. R. Dinkins, M. C. Freeman, C. R. Gilbert, A. G. Haines, D. C. Haney, G. Hill, M. H. Hughes, H. L. Jelks, T. Jones, J. M. Matter, P. W. Parmalee, J. Peterkin, C. E. Skelton, T. Smith, W. F. Smith-Vaniz, L. A. Somma, C. M. Timmerman, J. Troxel, and D. C. Weaver. M. C. Freeman kindly provided hydrographs and brought to our attention certain relevant literature. G. R. Dinkins and J.M. Pierson furnished many important recent fish records and accompanying collection data. J. T. Williams kindly loaned fishes from the U.S. National Museum of Natural History. K. W. Burkhead and L. G. Spinella generously assisted with editorial revisions. H. L. Jelks and D. C. Haney provided invaluable assistance in producing graphics, maps, and helping with editorial changes. M. C. Freeman and R. E. Sparks critically reviewed and greatly improved an earlier draft of the manuscript. Our work in the Etowah River system has benefited greatly over the years from discussions with R. G. Biggins, R. S. Butler, D. A. Etnier, M. C. Freeman, P. D. Hartfield, R. J. Larson, R. L. Mayden, and C. A.Williams. Lastly we thank J. A. Mann (Technical Information Service, National Biological Service) for always being pleasant, patient, and timely with our numerous literature requests. We regretfully apologize for inadvertently omitting others that have contributed substantially to this work
Gainesville Field Office: Lumpkin-Dawson-Forsyth, Federal Building, Rm. G-13, 126 Washington St. NE, Gainesville, GA 30501; phone 770 536-6981.
Rome Field Office: Polk-Floyd, 1401 Dean St., Suite F, Rome, GA 30161;
phone 706 291-5651.
Forest Service:
Chattahoochee/Oconee National Forest, 1755 Cleveland Hwy., Gainesville, GA 30501; phone 770 536-0541.
1720 Peachtree Rd., NW, Suite 816, Atlanta, GA 30367-9102; phone 404 347-4082.
Natural Resources Conservation Service:
1401 Dean St., Suite I, Rome, GA 30161-6494; phone 706 291-5652.
South Atlantic Division, U.S. Army Corps of Engineers, 77 Forsyth Street SW, Rm. 313, Atlanta, GA 30335-6801; phone 404 331-4619.
61 Forsyth St., Atlanta, GA 30303; phone 404 562-8327.
980 College Station Rd., Athens, GA 30605; phone 706 546-3136.
Water Resources Division, National Water Quality Assessment:
Regional Office: 3850 Holcomb Bridge Rd., Norcross, GA 30092;
phone 770 409-7700.Georgia District Office: Peachtree Business Center, Suite 130, 3039 Amwiler Rd., Atlanta, GA 30360; phone 770 903-9100.
Biological Resources Division, Florida Caribbean Science Center:
7920 NW 71st St., Gainesville, FL 32653; phone 352 378-8181.
4270 Norwich St., Brunswick, GA 31520; phone 921 265-9336.
1875 Century Blvd., Suite 400, Atlanta, GA 30345; phone 404 679-4000.
330 Richfield Ct., Asheville, NC 28806; phone 704 665-1195.
6620 South Point Dr., S., Suite 310, Jacksonville, FL 32216; phone 904 232-2580.
7 Martin Luther King Dr. SW, Suite 643, Atlanta, GA 30334; phone 404 656-4988
Floyd Towers East, 205 Butler St. SE, Suite 1058, Atlanta, GA 30334;
phone 404 656-4708.2070 U.S. Hwy. 278 SE, Social Circle, GA 30279; phone 770 918-6406.
745 Gaines School Rd., Athens, GA 30605; phone 706 369-6376.
7 Martin Luther King Dr. SW, Suite 643, Atlanta, GA 30334; phone 770 659-4905.
141 Willshire Rd., Rome, GA 30161; phone 706 295-6020.
Coosa District, 700 East 2nd Ave, Suite J, Rome, GA 30161; phone 706 295-6131.
4310 Lexington Rd., Athens, GA 30605; phone 706 542-3065.
3715 Northside Pkwy, 200 North Creek, Suite 300, Atlanta, GA 30327;
phone 404 364-2500.
No. 1 Jackson Hill Dr., Rome, GA 30163; phone 706 295-6485.
1310 West Ridge Rd., Gainesville, GA 30501; phone 770 538-2626.
650 North Main St., Suite A, Jasper, GA 30143; phone 706 692-5094.
503 W. Waugh St., Dalton, GA 30720; phone 706 272-2300.
135 West Cherokee Ave., Suite 251, Cartersville, GA 30120; phone 770 387-5030.
90 North St., Suite 310, Canton, GA 30114; phone 770 479-0501.
100 Cherokee St., Suite 300, Marietta, GA 30090; phone 770 528-3306.
P.O. Box 192, Dawsonville, GA 30534; phone 706 265-3164.
P.O. Box 946, Rome, GA 30162; phone 706 291-5110.
110 E. Main St., Suite 210, Cumming, GA 30130; phone 770 781-2100.
141 Pryor St., Atlanta, GA 30303; phone 404 730-8206.
99 Courthouse Hill, Suite A, Dahlonega, GA 30533; phone 706 864-3742.
120 East Memorial Dr., Dallas, GA 30132; phone 770 443-7514.
52 North Main St., Suite 201, Jasper, GA 30143; phone 706 692-3556.
P.O. Box 268, Cedartown, GA 30125; phone 706 749-2101.
2887 Alabama Hwy., Rome, GA 30165; phone 706 235-1043.
1930 Iris Dr., Conyers, GA 30207; phone 770 929-3350.
1776 Peachtree St. NW, Suite 400 South, Atlanta, GA 30309; phone 404 876-2900.
1330 W. Peachtree St., Suite 410, Atlanta, GA 30309; phone 404 873-6946.
1301 Sixth Ave., 31st Floor, New York, NY 10019; phone 212 841-4747.
2000 P St. NW, Suite 410, Washington, DC 20036; phone 202 293-1928.
333 Piedmont Ave., Bin No. 10230, Atlanta, GA 30308; phone 404 526-6784.
16 East 34th St., New York, NY 10016; phone 212 684-6577.
50 Hurt Plaza, Suite 1200, Atlanta, GA 30303; phone 404 522-6755.
Tallan Building, Suite 701, 100 West Martin Luther King Blvd., Chattanooga, TN 37402-2561; phone 423 756-0767
102 Reynolda Village, Winston-Salem, NC 27106-5123; phone 910 748-9222.
The Hurt Building, Suite 449, Atlanta, GA 30303; phone 404 688-5525.
1120 Connecticut Ave. NW, Bender Building, Suite 900, Washington, DC 20036; phone 202 857-0166.
50 Hurt Plaza, Suite 1200, Atlanta, GA 30303; phone 404 522-6755.
1290 Ave. of the Americas, New York, NY 10104; phone 212 373-4252.
One Commerce Sq., 2005 Market St., Suite 1700, Philadelphia, PA 19103-7017; phone 215 575-9050.
308 Mallory St., Suite C, St. Simons Island, GA 31522; phone 912 638-6265.
35 Wisconsin Circle, Suite 520, Chevy Chase, Maryland 20815; phone 202 783-8488.
One CNN Center, Suite 1090-South Tower, Atlanta, GA 30303; phone 404 681-9900.
232 East High St., Charlottesville, VA 22902-5178; phone 804 295-2134.