
In the historical perspective paper on aquatic mollusks included in this volume, Neves et al. (1997) review the diversity and current status of southeastern freshwater snails, clams, and mussels. The intent of our report is to review what has been done in past years to manage these freshwater mollusks and what could be done to manage them in the future.
This discussion uses an extremely broad definition of management as it applies to aquatic mollusk resources. We have chosen to include descriptions of all sorts of actual or potential activities which could lead to greater protection or enhancement of native aquatic mollusks. We have excluded purely taxonomic studies from this review because they rarely have advanced our understanding of the habitat, life history, or ecological requirements of these animals.
As you read on, it will become obvious that nearly all of this review deals with the large native freshwater mussels (Superfamily Unionoidea). Neves et al. (1997) indicated that several fingernail clams (Family Sphaeriidae) and many more freshwater snails (in the Orders Mesogastropoda and Basommatophora) occur in southeastern streams; however, as of yet, these groups have received little or no management attention.
On the other hand, a wide variety of information is available concerning freshwater mussel management, and the proceedings of two important mussel management meetings (Rasmussen, 1980; Cummings et al., 1993) have been extremely valuable in the preparation of this review. A new annotated bibliography on mussel reproduction and propagation (Watters, 1994) also has been extremely helpful in finding and organizing the contributions of various authors.
Given the regional focus of the symposium in which this paper was presented, this review of mollusk management activities has intentionally adopted a southeastern perspective. The work of southeastern researchers and projects in this region are used as examples whenever possible. This approach both carries out the symposium theme and indicates the relatively broad scope and depth of mussel research in this part of the United States. As we report, the diversity of aquatic mollusk problems and projects in the Southeast rival the diversity of the region’s species.
As indicated by Neves et al. (1997), rivers and streams in the southeastern United States support an extremely diverse snail fauna, at least as diverse as this region’s native mussel fauna. Southeastern aquatic snails have suffered from the alteration of streams and watersheds at least as much as the mussels (Stein, 1976; Neves et al., 1997). However, in spite of the losses of snail habitat and populations, little or no effort has been given to managing these resources. Protection of southeastern aquatic snails has not occurred, probably because none of the native snails have economic value; none but one are large or gaudy enough for most people to notice; and, until 1993, none had been listed as a federal endangered or threatened species.
In the absence of economic reasons, human interest, or regulatory requirements to protect them, the native freshwater snails have been left to the few naturalists and academicians who have become interested in them. Identification of many aquatic snail species is still extremely difficult either because so little careful attention has been given to the task or because these animals really do not follow our rules on the distinctions between species. At the moment, resource managers are faced with uncertainty regarding which snail species they should be concerned about, and they have no compelling reasons to spend either time or money on any of them.
There is one obvious exception to these generalities about freshwater snails. The spiny riversnail, Io fluvialis, is the largest gastropod found in North American rivers and streams. The snails presently grow up to 50 mm (about two inches) in length. As the common name implies, many individuals of this species have obvious conical spines on their shells which make them look like out-of-place marine murex or whelks. Natural populations of this species survive only in parts of the Powell, Clinch, and Nolichucky rivers in Tennessee and Virginia, but the species’ former range extended down the mainstem Tennessee River to near Muscle Shoals, Alabama (Stansbery and Stein, 1976). Tennessee River Io specimens, which were as much as 60 mm (about 2.4 inches) long and very obviously spined, often wash or are dug out of Native American refuse heaps along the river banks. Years ago, the spiny river snail was adopted as the symbol of the American Malacological Union, and since 1977 this species has been a candidate for federal listing as an endangered or threatened species (U.S. Fish and Wildlife Service, 1977a).
The reason for bringing Io fluvialis into this discussion is that the species has benefited from an intentional management effort. In 1978 and 1979, Ahlstedt and other Tennessee Valley Authority (TVA) staff transplanted spiny river snails from the Clinch River to sites on the North Fork Holston River (Ahlstedt, 1991). That Holston River tributary once had supported an Io population, but those snails and most other aquatic species had been extirpated by discharges from an upstream chemical plant. After the plant ceased operation and the site was reclaimed, TVA had documented that life in the river was starting to recover. Sampling near the North Fork Holston River transplant sites since 1979 has indicated a steady increase in spiny river snail numbers and the populations are beginning to spread in the river (Ahlstedt, 1991). This appears to be the first intentional transplant of an American aquatic snail species, and it also represents a documented management success. Apparently, we know at least one method of managing aquatic snails but, so far, do not have the incentive to take on the task.
Most members of the general public know very little about the large freshwater mussels which are native to Interior Basin and southeastern streams. However, within a fairly small community of mussel biologists, commercial fishermen, game managers, exporters, and jewelers, freshwater mussels constitute an extremely important American resource. From various perspectives, native mussels have substantial commercial value and ecological significance. Both of these reasons have led to activities intended to protect, preserve, or enhance mussel resources (our definition of resource management).
Beginning with the earliest contacts with Native Americans, European explorers have known that attractive and potentially valuable pearls could be found in North American mussels (Dickinson, 1968). Unfortunately, the chances of discovering valuable pearls in native mussels have always been extremely low. Also, in the absence of a nearby market, the value of pearls was often established by someone willing to buy them. Waves of pearl hunting have occurred rather consistently all across the eastern United States since colonial times (Kunz, 1898b). The pearl hunters and buyers had an obvious interest in native mussels as the source for what they sought; however, that interest did not result in any documented attempts to manage mussel stocks.
Around 1890, George Boepple, a German immigrant, recognized that the shells of many North American mussel species were extremely good raw materials for making inexpensive, durable buttons (O’Hara, 1980). He adapted European button technology to use this new resource and started making pearl buttons near Muscatine, Iowa. Within the decade, pearl button factories were in operation all along the upper Mississippi River. Almost as quickly, mussel resources adjacent to the button factories were depleted and a nationwide market developed for high-quality button shells. The annual commercial harvest of button shells jumped from literally zero in the late 1880s to thousands of tons by 1910 (Figure 1).
After World War II, plastic buttons became available and the pearl button industry
declined and disappeared, only to be replaced by another commercial use for
mussel shell. The cultured pearl industry developed in post-war Japan and, since
the 1960s, has become a major international enterprise (Ward, 1985). North American
mussels are crucial to this industry because beads made from these shells are
tolerated by marine pearl oysters and retain the thin layers of pearly material
which the oysters deposit on them. The market for native mussel shells created
by the cultured pearl industry has increased both the number of commercial mussel
fishermen and the harvest pressure on surviving mussel populations (Figure 2).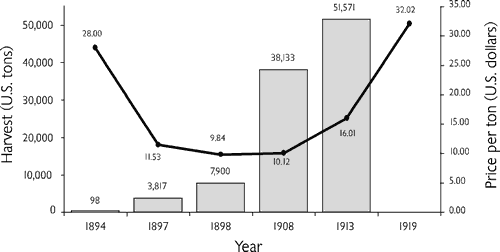
A very different reason for managing native mussel resources began in 1976 when the U.S. Fish and Wildlife Service (USFWS) added the first mussel species to the federal list of endangered wildlife (U.S. Fish and Wildlife Service, 1976). Subsequent research has demonstrated that major mussel habitats have been severely modified and important components of the native mussel fauna are near extinction. Sections of the U.S. Endangered Species Act require federal agencies to determine if their actions will further jeopardize listed species. More broadly than that, the public interest which has been generated as species have been added to the endangered or threatened lists has encouraged many individuals and organizations to investigate why so many species are in peril and what can be done to prevent or reverse the threat of extinction.
The various types of activities which have been implemented or proposed to protect and enhance mussel resources could be presented in several different ways. The following semi-historical approach indicates both the ideas which have been proposed and the context in which they developed.
In the mid-1890s, less than five years after the button industry started, mussel resources in the Mississippi River near the Muscatine, Iowa, factories had become obviously depleted. Button manufacturers requested assistance from the U.S. Fish Commission (later called the U.S. Bureau of Fisheries and, later still, the U.S. Fish and Wildlife Service). This request started a 40-year federal program of research and scientific inquiry focused on commercially important native mussels.
As this program began, the existing status of the resource and commercial interest
in native mussels were documented in three Bureau of Fisheries reports. Apparently
without leaving New York, George F. Kunz, a well-known gem and pearl expert,
produced both a short and a long report on pearls and pearl fisheries in the
United States (Kunz, 1898a, 1898b). Charles T. Simpson, curator of mollusks
at the U.S. National Museum, wrote a non-technical description of the anatomy,
ecology, life history, and problems facing native mussels in the United States
(Simpson, 1899). Hugh M. Smith, then a fisheries biologist with the Bureau,
went to the button manufacturing area and produced a detailed report on the
mussel fishery and pearl button industry as it existed at the time (Smith, 1899).
Regardless of its short length, the Simpson report (loc. cit.) clearly presented
what was then known about mussel biology and life history. Information contained
in both the Simpson and Smith reports (both loc. cit.) presented their initial
concepts about management activities required to sustain the commercial fishery
and protect mussel resources.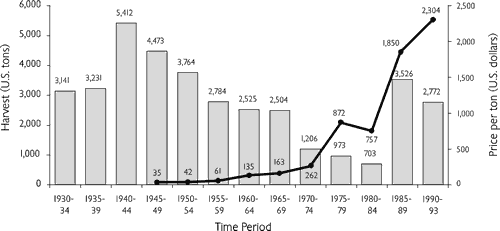
During the next four decades, the Bureau of Fisheries supported a wide range of research projects in four broad categories: biology and life history studies, resource surveys, propagation work, and identification of protection measures. Initially, much of this research was conducted at the Fairport Biological Station located along the Mississippi River near the center of the button industry. The program quickly grew, however, to include more and more rivers across the nation.
Life History Studies
State-of-the-art biological and life history studies (e.g., Figure 3) were
conducted by a variety of individuals in close association with others working
under different sponsorships. Between 1900 and 1920, Bureau-supported studies
confirmed that most native mussels had separate sexes (Lefevre and Curtis, 1912)
and that shell rings could be used as approximate indicators of age (Isley,
1914). Other Bureau projects demonstrated that mussel larvae (glochidia) could
be identified by their size and shape (Surber, 1914, 1915) and that the larvae
of almost all species had to spend some time as obligate parasites, generally
on fish, before they could develop into adults (Lefevre and Curtis, 1912). The
findings of many Bureau studies were summarized in a non-technical report (Coker
et al., 1921) which made much of this information available to the button industry
and state resource managers. After 1921, Bureau-supported researchers working
on life history projects focused on narrower topics such as mussel feeding mechanisms
and food (Churchill and Lewis, 1924) and comparisons of growth rates at different
locations (Chamberlain, 1931). Most of what is known today concerning the ecological
and life history characteristics of native mussels was discovered during these
Bureau of Fisheries studies.
Resource Surveys
Biologists working on mussel projects for the Bureau of Fisheries had obvious interest in where mussel stocks existed because of their close association with the constant demands of the button industry. Once mussel resources on the upper Mississippi River were depleted, mussel fishermen and Bureau biologists literally searched the nation for high-quality button shells. Between 1912 and 1915, Bureau of Fisheries reports were published on the status of mussel stocks in over 15 important watersheds or larger regions of the nation. In addition to documenting the quality of commercial mussel stocks present at that time, reports such as "The Mussel Resources of the Illinois River" (Danglade, 1914) and "The Mussels of the Cumberland River and its Tributaries" (Wilson and Clark, 1914) provided distribution and abundance benchmarks to which many subsequent studies have been compared.
Propagation Work
From the beginning, the chief purpose of the Bureau of Fisheries’s work on native mussels was to find ways to replace stocks of species harvested to make buttons. After it was confirmed that virtually all larval mussels must spend some time as parasites on fish before they could develop into adults, Lefevre and Curtis began a long series of experiments on ways to increase recruitment success. The essential features of their artificial propagation technique were published in 1912 (Lefevre and Curtis, 1912) and, by 1921, had become a standardized procedure (Coker et al., 1921). Each year from some time before 1919 (Smith, 1919) until 1930 (Jones, 1950), Bureau employees infected thousands of fish with millions of glochidia before releasing them into the Mississippi and many other rivers. Fish infections were stopped for several years during the 1930s, but the techniques were improved and infections were resumed to some degree at least as recently as 1950 (Jones, 1950).
In addition to the release of infected fish, Bureau staff also began to culture juvenile mussels which had been transformed on fish (Figure 4). Experiments on juvenile mussel culture were described by Howard (1922) and at least one mass transplant of juvenile mussels was made into Virginia waters in 1926 (Higgins, 1928).
Interest in both fish infections and juvenile mussel culture was reduced substantially in the late 1920s because new considerations were coming into play. In 1926, Ellis used experimental work on mussel blood as a starting point in developing a way to transform glochidia into juvenile mussels without the use of a fish host (Ellis and Ellis, 1926). This artificial propagation of mussels offered the possibility of rearing large numbers of juveniles without having to deal with difficult and, for some mussel species, unknown fish hosts.
In the late 1920s, the emphasis of Bureau mussel work was focused on perfecting this artificial culture technique, and some laboratory-scale successes were reported (Higgins, 1930, 1931). Very soon, however, Ellis and other Bureau staff found that protozoans and pollution were severely reducing the percentage of viable glochidia in female mussel gills. Field studies conducted in the Mississippi and other major rivers indicated that although protozoans occurred in many locations, siltation and pollution were affecting much larger amounts of previous mussel habitat (Ellis, 1931a). While Bureau-sponsored research on artificial propagation continued for several years (at Columbia, Missouri and Fort Worth, Texas), Ellis and associated staff shifted their focus to exploring the broad range of pollution and sedimentation effects on aquatic life. By 1938, the Bureau of Fisheries annual report on scientific research excluded any mention of mussel propagation activities (Higgins, 1939).
Protection Measures
Also from the beginning, biologists working with the Bureau of Fisheries attempted to encourage the states and commercial interests to protect existing mussel stocks. Both of the initial Bureau studies on mussels (Simpson, 1899) and the button industry (Smith, 1899) included recommendations for actions which would help prevent further depletion of mussel resources. Smith prefaced his list of recommendations with a clear statement of where the authority to implement any protection measures resided:
"… It should, however, be understood that the perpetuation of this important industry depends wholly on the joint action of the States concerned, and that the General Government and the U.S. Fish Commission are entirely without jurisdiction" (Smith, 1899; page 313).
The recommendations proposed by Smith included minimum size restrictions (by
species), closed seasons (when mussels were spawning), establishment of pollution
controls, prohibitions on interstate shipment of shells, and reduction of shell
waste during button production.
Fifteen years later, another Bureau biologist offered a second set of recommendations on protecting mussel resources (Coker, 1914). By this time the Bureau had completed many of its surveys and life history studies and had started stocking infected fish in a number of areas. Bureau staff also had come to realize that any results achieved through propagation could be quickly undone by strong harvest pressure, especially in the absence of state protection measures. Coker recommended the imposition of a single minimum size limit for all species (two inches [about 5 cm] in greatest dimension), rotating closure of large river reaches for several years to allow mussel stocks to recuperate, and state enforcement authority. He also suggested the states adopt a mussel fishing license fee to cover the cost of enforcing compliance with these laws. In 1919, the Bureau assisted in drafting model legislation for the states to encourage uniform and adequate protection of mussel resources (Smith, 1919).
In 1931, results from the propagation and pollution research led the Bureau of Fisheries to radically modify their recommendations to the states. Ellis and his coworkers had concluded that "Extensive and rapid reduction, amounting in many places almost to extermination, of the mussel fauna is to be expected if the erosion and pollution problems are not solved" (Ellis, 1931b; page 10). Acting on these findings, the Bureau recommended the states eliminate all restrictions on mussel harvesting so that existing mussel stocks could be used before they were lost (Carlander, 1954). While some lingering Bureau of Fisheries mussel research projects continued for a few more years and a few commercial mussel projects were conducted later (see below), this conclusion and recommendation signaled the end of extensive Bureau-sponsored research on commercial mussel species.
During the early years of the button industry, in spite of the recommendations from the Bureau of Fisheries, most states did not attempt to regulate the mussel harvest. By the late 1910s, however, many mussel-producing states recognized that mussel stocks were severely depleted and they began implementing harvest regulations (Waters, 1980). Those states, most of which were located along the upper Mississippi River, instituted license fees, set minimum size limits, and restricted harvest gear. Some closed areas to harvest for a time. Although some improvements in mussel stocks were observed in a few areas, all stocks generally continued to decline. In light of these failing efforts and the emerging understanding of siltation and pollution effects on mussels, in 1931 when the Bureau recommended elimination of all harvest restrictions, most states quickly adopted the new position to use the remaining resource rather than waste what would soon be gone anyway.
Between the mid-1930s and early 1960s (or in some instances the 1970s), very few states paid much attention to mussel resources or the mussel harvest. A few states, such as Kentucky, continued to license mussel fishermen, required annual harvest reports, and took other actions to protect commercial mussel resources (Crowell and Kinman, 1993). Most states realized that the button industry was declining but did not appreciate the impact of the new market for shells to supply the cultured pearl industry.
Another change which occurred between the 1930s and 1970s was the substantial modification of nearly all the large rivers which once supported extensive mussel populations. By the late 1970s, hydropower facilities had been constructed at practically every feasible site on the continent’s mainstem rivers except in Alaska and northern Canada (Stanford and Ward, 1979). Initial assumptions were that low flow conditions and increased sedimentation would eliminate all mussel stocks in the various impoundments (Ellis, 1931a). When mussel harvesting resumed after World War II and pre-impoundment mussel stocks were found to persist in many areas, those assumptions were presumed to be incorrect. For several years, the commercial harvest increased to meet a growing pearl culture demand, but price also increased as high-quality shell became more difficult to find (Figure 2).
In the early 1950s, the struggling pearl button industry once again requested federal assistance to investigate the status of the mussel resource and recommend measures to increase stocks of valuable mussels (Scruggs, 1960). The USFWS conducted a two-year study of mussel stocks at selected sites on the Tennessee River which, since the war, had become the most important source of freshwater shell. That study concluded that populations of the most important commercial species (Pleurobema cordatum) were being extensively harvested but were experiencing almost no recruitment, possibly because impoundment had made radical changes in big-river habitats (Scruggs, 1960).
In 1963, with the encouragement of the states and several shell companies, TVA conducted an evaluation of mussel stocks all along the mainstem Tennessee River in Kentucky, Tennessee, and Alabama (Isom, 1966, 1969). Results of that evaluation indicated that suitable mussel habitat persisted only in 33 percent of the river reach studied. In those areas, overharvesting was causing a rapid depletion of the mussel resources. TVA recommended the three states adopt regulations to control the harvest, establish sanctuaries where harvesting should not occur, and conduct life history studies to find out why important commercial species were not reproducing (Isom, 1966).
This time the states were quick to respond. In 1965, Kentucky restricted mussel harvesting to daylight hours, defined legal harvest methods, imposed a minimum size limit of 2.5 inches (6.35 cm), and created a sanctuary in the Tennessee River immediately downstream from Kentucky Dam (Crowell and Kinman, 1993). Also in 1965, Tennessee passed enabling legislation to issue licenses and began regulating the mussel harvest. Those regulations included license requirements for both mussel fishermen and buyers, a size limit of 2.5 inches (6.35 cm), gear restrictions, daylight harvesting only, and establishment of sanctuaries (Todd, 1993). In 1966, Alabama passed enabling legislation and started regulating its mussel harvest along similar lines (Isom, 1969).
By the mid-1970s, the upper Mississippi River states were conducting mussel surveys to determine the status of their remaining mussel stocks. These surveys were prompted by concerns over poor water quality conditions, ongoing channel maintenance activities, levee construction, and likely overharvest of the mussel resource.
In 1985, the five upper Mississippi River states met to determine what actions were necessary to prevent further resource declines. These managers recommended increasing the minimum legal size limits, reducing the length of the harvest season, and implementing a harvest reporting system. They also recommended that the states try to make their regulations as uniform as possible. Most of these recommendations were implemented by the states by 1992 (Thiel and Fritz, 1993).
The middle states of the Mississippi River Valley also were reevaluating mussel management policies during the later 1980s. Reports of mussel die-offs and increased harvest pressure stimulated these states to obtain information regarding their mussel resources. Most states did not have the necessary information regarding their mussel stocks to make informed management decisions, and many states decided to close their waters to mussel harvest until such information could be collected. Kentucky, Oklahoma, and Tennessee closed some productive areas to commercial musseling to slow the harvest and protect the resource. Tennessee also funded surveys to determine the current state of its mussel resources (Bates and Dennis, 1985).
In the early 1990s, many states implemented more restrictive regulations regarding commercial musseling. Several states increased the minimum size limits on commercial species, limited which species could be harvested, closed waters thought to be experiencing overharvest, shortened the harvest season, instituted mussel harvest reporting procedures, restricted the types of gear which could be used for mussel harvest, and limited access to the fishery. These more restrictive regulations were aimed at protecting mussels until they were old enough to reproduce, reducing the take of non-marketable shells, minimizing the impact on non-target species, providing data on the harvest trends, and reducing the number of fishermen (Todd, 1993).
Management interest in native mussels probably would have remained focused only on species with commercial value if mussels had never been added to the federal lists of endangered or threatened species. However, some native mussels have been declared endangered species, and the management of mussel resources has changed tremendously because of this.
The U.S. Endangered Species Act (ESA) was passed in 1973. In addition to establishing formal lists of endangered and threatened wildlife and plants, this law requires federal agencies to ensure that activities they conduct, fund, or authorize do not jeopardize the continued existence of species on either the endangered or threatened lists. The law also establishes procedures for states to receive federal funds to protect and enhance listed species (U.S. Fish and Wildlife Service, 1992).
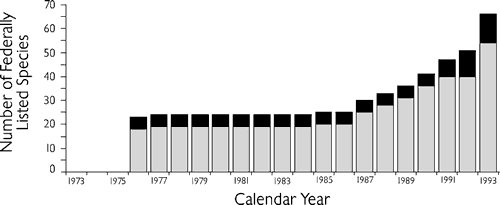
The first group of native mussel species was added to the endangered species list in 1976 (U.S. Fish and Wildlife Service, 1976). Eighteen of the 23 species in that group occur in one or more of the southeastern states. One additional mussel species was listed as endangered in 1977; it also occurs in southeastern states (U.S. Fish and Wildlife Service, 1977b). Other mussels were not added to either the endangered or threatened federal lists until 1985; however, there has been a steady stream of listings since then (Figure 5).
The federal listing of 24 endangered mussel species had a dramatic effect on several federal and state agencies, especially in the southeastern states. Because of their roles in developing and maintaining water projects, the U.S. Army Corps of Engineers and TVA now had new responsibilities: first, to find out whether endangered mussels (and other listed species) were present where projects were being proposed and, second, to determine what they should or could do to avoid impacting those species. The USFWS, charged with implementing the ESA inland, also needed up-to-date distribution and life history information to protect each listed species and enough expertise in impact assessment to evaluate the proposals being made by other federal agencies.
State agencies were faced with a slightly different situation. A wide variety of projects which involved federal funds or required federal permits were now being evaluated for impacts on species (mussels as well as other groups) that many state biologists knew little or nothing about. In addition, federal biologists or their contractors were surveying streams all across the states, gathering information on mussels, fish, and other species which had been studied very little because they had no commercial or sport value. Beyond that, the ESA provided a new source of federal funds to study local fish and wildlife, once appropriate state programs were established.
This new emphasis on all native mussels caused by the ESA listings spawned a variety of research efforts not unlike those started by the Bureau of Fisheries almost a century before. The primary difference associated with this new wave of research on mussel life histories, distribution surveys, propagation efforts, and protection measures was that these studies were focused on rare or non-commercial species. In addition, these projects were not being conducted by a single organization, but by individuals working for a variety of organizations, often with different missions.
Life History Studies
While there had been a steady trickle of mussel life history studies since the 1930s (e.g., Stein, 1968; Yokley, 1972), the pace of this work started to increase in the late 1970s, and the focus shifted toward endangered species. Between 1981 and 1985, TVA biologists conducted a series of fish infection experiments focused on identifying hosts for two endangered mussel species. The results of that work (Hill, 1986; Yeager and Neves, 1986) established one or more hosts for seven mussel species, including two listed endangered species and three species which were candidates for listing. Neves and students working at Virginia Polytechnic Institute started fish host identification studies about the same time and, so far, have identified hosts for at least ten mussel species (Neves, 1991; Watters, 1994). Others also have started filling this information gap for different species (e.g., Parker et al., 1984; Buchanan, 1987; Gordon and Layzer, 1993).
In addition to fish host identifications, a number of recent projects or observations have explored other mussel life history and ecological relationships. During the early 1980s, TVA spent a substantial amount of time and effort attempting to identify the essential characteristics of endemic (Cumberlandian) mussel habitat (Jenkinson and Heuer, 1986). Others have begun to explore a variety of new topics, including juvenile mussel habitat preferences (Yeager et al., 1993), special reproductive behaviors of gravid females (Buchanan, 1987), and population dynamics (Payne and Miller, 1989).
Distribution Surveys
Updating distribution and abundance information have been essential components of the new emphasis on all mussel species. Only a few workers had conducted detailed distribution surveys since the 1930s (e.g., Isom and Yokley, 1968; Starrett, 1971), and the status of the entire fauna was extremely poorly known. Starting in the late 1970s, relatively traditional surveys of smaller streams, such as the Meramec River in Missouri (Buchanan, 1980), several rivers in Mississippi (e.g., Hartfield and Jones, 1990), and smaller rivers in the Tennessee Basin (Ahlstedt, 1986; Jenkinson and Ahlstedt, 1988), have provided updated information on mussel populations in many streams. The widespread availability of scuba and other diving equipment in recent years also has made it possible to conduct detailed surveys of mussel resources in the largest rivers (e.g., Pardue, 1981; Sickel, 1985).
Propagation
A new wave of interest in mussel propagation began in 1982 when a TVA biologist and contractor announced they had used an artificial medium to produce juvenile mussels without the involvement of a fish host (Isom and Hudson, 1982). While it is still not possible to turn a gill full of mussel larvae into 50 or 100 thousand yearling mussels ready to be stocked in some stream, this potential has led to a variety of research projects using juvenile mussels (e.g., Dimock and Wright, 1993). The ability to produce known-age juveniles of at least one species also has made it possible to start conducting standardized toxicity tests on native mussels (Wade, 1990). The results of such studies are beginning to indicate that native mussels have very different tolerances to many pollutants than the standard fish or arthropod test organisms (Wade et al., 1993).
Protection Measures
Protection and, more recently, enhancement measures for the full spectrum of native mussel species now include a variety of approaches. Resource management agencies have reduced the number of species which can be harvested to those most important to the industry and have restricted the harvest to those areas which can withstand the losses (e.g., Todd, 1993). Some states are carefully selecting minimum size limits which will allow animals to spawn before they can be harvested. Mussel sanctuaries have been established in areas with few commercial species, in part to minimize impacts on non-commercial species. Information guides produced by states and others help mussel harvesters learn more about the legal species and mussels in general. Strategies are now being pursued by some states to establish and maintain a stable mussel harvest without affecting the long-term survival of all mussel species (e.g., Hubbs, 1995).
Federal agencies also have begun to take protection and enhancement action in a variety of different ways. Recovery plans for endangered and threatened species, prepared or sponsored by the USFWS, have highlighted research and/or enhancement needs (e.g., U.S. Fish and Wildlife Service, 1984). In 1992, TVA started improving habitat conditions for mussels and other aquatic life downstream from its tributary dams by adopting minimum releases and by beginning to correct seasonal low dissolved oxygen levels (Davis and Brock, 1994). Specific habitat improvements (Miller, 1983) and reintroductions (Jenkinson, 1983; Sheehan et al., 1989) have been made to restore or accelerate the recovery of degraded mussel communities. A number of agencies and organizations are beginning to work together to address various types of nonpoint-source pollution to restore degraded stream water and habitat quality (Water Quality 2000, 1994). Native mussels, especially endangered species, are often the intended benefactors of these activities (Master, 1993). The USFWS also has enhanced public education and interest in native mussels by sponsoring the production of a high-quality videotape and a poster about them (Helfrich, 1993; Helfrich et al., 1993).
Anyone unfamiliar with the current status of freshwater mollusks might assume the preceding history of commercial mussel management activities and new emphasis on non-commercial species should mean that native mussels, if not all freshwater mollusks, are now being carefully managed and protected from further harm. Unfortunately, that is not the case. Results of various studies conducted during the last 20 years indicate that commercial species and many non-commercial mussels are still declining, both in terms of their distributions and numbers of individuals (Williams et al., 1993).
Two recent evaluations present similar views on the current status of North American mussel resources. In a 1992 "A State-of-the-Unionids Address," Neves concluded the following:
"The freshwater mussel fauna of the United States is in serious trouble. Of the 297 species and subspecies recognized, 21 species (7%) are presumed extinct, 42 (15%) are federally listed as endangered or threatened, and 69 (23%) are candidates for federal protection. The highly diverse endemic mussel fauna of the southeastern United States is in greatest jeopardy, with depressed population levels today reflecting transgressions to rivers decades earlier" (Neves, 1993; page 1).
Williams et al. (1993) reported similar statistics for the United States and Canada. They also summarized the existing threats and likely future for mussels as follows:
"The primary reasons for the decline of freshwater mussels are habitat destruction from dams, channel modification, siltation, and the introduction of nonindigenous mollusks. The high numbers of imperiled freshwater mussels in the United States and Canada . . . portend a trajectory toward an extinction crisis that, if unchecked, will severely impoverish one of our richest components of aquatic biodiversity" (Williams et al., 1993; page 6).
If the native freshwater mussels and other aquatic mollusks are to survive, several types of management activities will have to be conducted. From our perspective, four broad categories of such need to be addressed: habitat protection, population enhancements, harvest controls, and public appreciation. Each of these categories is briefly explored below.
It is becoming increasingly clear that the presence of a diverse, reproducing mussel community indicates that important physical, chemical, and biological features of the habitat have been stable for a long time, at least several decades. The protection of existing native mussel communities will require the identification and perpetuation of the full set of habitat features which are essential to their survival. If mussel populations or communities are to be restored in an area, these essential habitat features will have to be present and expected to persist for decades.
At the present time, we know the general concepts but lack specifics on the habitat features which are essential for mussel species survival. Careful, broad spectrum evaluations of good mussel habitats should help identify these features, especially if the results can be compared to a variety of habitats where diverse mussel communities no longer exist. Pioneering habitat studies conducted by TVA (Jenkinson and Heuer, 1986) and Layzer (e.g., Cochran and Layzer, 1993) appear to be valuable examples of such research; however, much more work on this topic is urgently needed.
Even before there is reasonable assurance that suitable mussel habitat will continue to be available, a variety of techniques must be developed to protect or augment mussel species reduced to very few populations or individuals. If possible, fish hosts should be identified for all species so that essential habitat features for both the mussels and these fishes can be protected. Where required combinations of habitat features are present, fish and mussel life cycles are very likely to continue without the need for human intervention.
When the surviving stock of a mussel species is extremely small or does not appear to be reproducing in nature, some form of artificial propagation will have to be used to save that species from extinction. Results from various propagation experiments suggest that it should be possible to transform the larvae and raise the juveniles of most mussel species. However, there are still a number of improvements which must be made before these techniques can be expected to work consistently. If a large-scale artificial propagation technique were to become available, a tremendous variety of restoration, enhancement, genetic study, and commercial possibilities could begin.
Regardless of whether artificial propagation techniques are perfected soon, it may be necessary to develop ways to store mussel genetic and reproductive material before some species are lost completely. Genetic material, gametes, and embryos of other animals and plants have been frozen and revived to develop normally (Ballou, 1992). If appropriate cryopreservation techniques could be developed for mussels and other aquatic mollusks, the unique characteristics of species and populations could be saved for possible future study and, hopefully, for eventual reintroductions into streams.
State agencies are becoming increasingly more knowledgeable about the biology of various mussel species sought by commercial fishermen. If the commercial fishery is to survive, habitats and mussel populations will have to be carefully managed to balance both the needs of the mussels and the desires of the harvesters. To achieve such a balance, the states will have to monitor recruitment and growth rates of current and potential commercial stocks as well as the extent and intensity of the commercial harvest. Management activities based on these data could include selective closing of areas to harvesting, varying legal minimum size limits on particular species, and imposing substantial financial and license penalties for illegal activities. Adoption of uniform size limits by all states would simplify law enforcement activities and reduce confusion among the commercial harvesters.
None of the preceding management activities is likely to be conducted or to be particularly successful if it does not have at least some level of public support. Native mussels (and other aquatic mollusks) are not cuddly, generally appreciated, or fun to watch. Their abundance or absence in a stream, however, can and should convey an important message about the quality of that water. Given the sizable, and growing, human population in the southeastern states, the resident aquatic mollusks may not survive much longer in the absence of substantial public support. Interestingly, current information also suggests that human populations in the southeastern states may not have sufficient potable water to meet their future needs if the deterioration of rivers and streams is not stopped.
Federal agencies, state agencies, and other organizations must make the public more aware of the value of aquatic diversity, especially as it relates to water quality. Public comments about faunal posters, environmental brochures, nature talks, and the like suggest that people are willing to appreciate and support resource management once they learn of its relevance. Attractive nature posters, interesting videotapes, and engaging exhibits can expose people to the unique communities which exist in their local creeks. Well-written brochures, carefully planned demonstrations, and involving workshops can provide non-professionals with the information they need to evaluate land- and water-use activities on their own. A coordinated, long-term awareness program could turn passive public curiosity about what is in the water into widespread support for the protection and restoration of aquatic mollusks as residents of valuable stream ecosystems.
If all four of the preceding categories of management activities were implemented and nothing else interfered, southeastern native mollusks and the natural communities in which they live would survive and flourish. The management categories just described all consist of activities which agencies or groups of individuals could conduct, if they possessed enough resolve, time, and effort. Unfortunately, there is another threat to southeastern mollusks over which we have little or no control. That threat is two closely related species of zebra mussel (Dreissena polymorpha and D. bugensis). These bivalve mollusks were introduced into North America in the mid-1980s, probably from one or more locations near the Caspian Sea. Zebra mussels are spread as larvae floating in the water or as adults attached to boats and barges. As adults, zebra mussels live attached to virtually any firm substrate and, where habitat conditions are favorable, they can occur by the thousands per square meter (Claudi and Mackie, 1994).
The chief threat zebra mussels pose to native mollusks is that they readily attach to exposed parts of mussel and snail shells, often in such large numbers that they interfere with normal feeding and movement activities. In Lake Erie, where zebra mussels were first found in 1988, native mussel populations have been virtually extirpated by zebra mussel encrustations (Haag et al., 1993).
Zebra mussels were first observed in the Southeast in the lower Tennessee River in 1991. By 1993, the species had been spread up the Tennessee to Fort Loudoun Dam (near Knoxville) and down the Mississippi River to near New Orleans. Projections are that zebra mussels will eventually spread to virtually all of the large, navigable rivers in the Southeast (Strayer, 1991). If that happens, and if zebra mussels establish large populations, they could destroy the remaining mussel and snail populations in those southeastern rivers. Zebra mussels also are likely to eventually spread to tributary reservoirs and smaller streams. Whether such populations will become large enough to impact the native mollusks in those areas is not yet clear.
At present, there do not appear to be any significant predators, other natural controlling agents, or artificial ways of suppressing zebra mussel populations which would not harm components of natural aquatic communities. Once zebra mussels are introduced to an area, we can only wait and watch to see what impacts they will have. In at least some areas, large zebra mussel populations may extirpate the few native mollusks which have survived the habitat modifications, harvest pressure, and other assaults we have inflicted.
This review of management activities affecting native aquatic mollusks, primarily freshwater mussels, has covered considerable calendar time and subject matter. American interest in protecting and augmenting native mussel stocks began approximately 100 years ago when the shells of some mussel species were recognized to have commercial value as the raw material used by the pearl button industry. Today, mussel shell is still an important raw material, except now shell beads are used as nuclei for cultured pearls.
Twice during this 100-year period considerable emphasis has been given to research on native freshwater mussels: first between about 1900 and 1930 when button shells were becoming increasingly hard to find and, second, starting in the late 1970s after passage of the U.S. Endangered Species Act. Both times, the research effort focused on learning more about the habitat requirements, life histories, and existing distribution of various mussel species. Both times, new propagation techniques were developed and new protection measures were devised to prevent and, hopefully, reverse the further depletion of existing mussel stocks.
Today, native aquatic mollusks seem to be closer to extinction as a group than ever before. Commercial mussel resources are still harvested; however, decreasing mussel populations and increasing prices have combined to reduce the acceptable options available to both the harvesters and the state regulatory agencies. Non-commercial mussel and, probably, most snail species now exist only in small percentages of their former ranges, generally where the habitats have survived extensive degradation by sedimentation and impoundment. In many of these stream habitats, resident mollusk populations are declining, but the specific reasons for the declines are not known. Most federal and state agencies, especially in the Southeast, are aware of the plight of these mollusk resources, but the general public is barely aware that these animals even exist.
A variety of research and management actions have been identified which could help protect and restore native aquatic mollusks. More precise identification of the specific habitat features important to various mussel species could lead to the protection or restoration of stream reaches where mollusks and other members of diverse aquatic communities could survive. Identification of mussel fish hosts and the perfection of artificial propagation techniques could help insure the survival of existing mussel populations and provide ways to prevent the extinction of species reduced to only a few individuals. Adoption of biologically based harvest regulations could protect mussel populations while providing a sustainable level of mussel harvest. Public education programs, supported and coordinated by a variety of organizations, could help citizens to realize that the current plight of native mollusks should be viewed as a warning about the potential loss of stream ecosystems and all of the species they support, including us.
During the last 100 years, interested Americans have learned a great deal about the native mollusk resources in our streams and what we should do to protect and enhance them. Those management activities, if implemented, could prevent species extinctions, restore commercial stocks, and improve the overall quality of the water we use. Several agencies are already working to make this happen and, with increased public support, success could be possible. But, even then, our native aquatic mollusks would still be threatened by an exotic biological threat, the zebra mussel invasion.